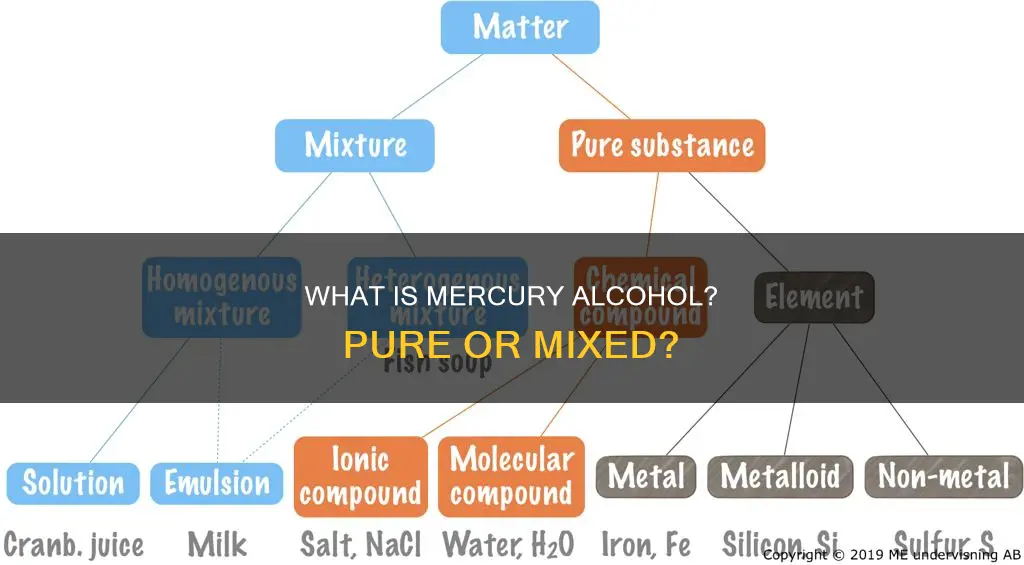
Mercury and alcohol are two very different substances with distinct properties. Mercury is a chemical element, a silvery liquid metal at room temperature, and is denoted as Hg on the periodic table. Alcohol, on the other hand, is a compound, a product of the chemical bonding of hydrogen and oxygen atoms, and is commonly found in beverages, fuels, and solvents. The question of whether mercury alcohol is a pure substance or a mixture requires an understanding of the definitions of these terms. A pure substance has a consistent composition, while a mixture combines different substances that retain their individual properties.
| Characteristics | Values |
|---|---|
| Pure substance | Yes |
| Mixture | No |
| Consistency in composition | Yes |
| Combination of different substances | No |
| Type of atom | Single (Hg) |
| Type of molecule | Single (C2H5OH) |
| Boiling point | Constant |
| Density | Constant |
What You'll Learn

Pure ethanol consists of C2H5OH molecules
Pure substances are defined as materials with consistent compositions, while mixtures are combinations that retain individual properties. Pure ethanol, also known as ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a type of alcohol found in alcoholic beverages and modern thermometers. It is a colourless, volatile, and flammable liquid that is soluble in water. Its chemical formula is C2H6O, or can be written as C2H5OH or CH3CH2OH. Pure ethanol consists of C2H5OH molecules, which contain two carbon atoms, five hydrogen atoms, and one oxygen atom.
Ethanol's hydroxyl group causes the molecule to be slightly basic. It has a pH of 7.33, which is almost neutral like water. The hydroxyl group also enables ethanol to participate in hydrogen bonding, making it more viscous and less volatile than less polar organic compounds of similar molecular weight. This hydrogen bonding also makes ethanol hygroscopic, allowing it to readily absorb water from the air. Due to the presence of both polar and nonpolar ends in the ethanol molecule, it can dissolve a variety of polar and nonpolar substances, including ionic compounds and nonpolar substances such as essential oils and colouring agents.
Ethanol is produced through the fermentation process, where sugar molecules are broken down by yeast. It has a wide range of applications, including its use as a solvent in personal care products like perfume, mouthwash, and deodorant, as well as in food colouring and additives for flavouring. Additionally, ethanol can be found in hand sanitiser gels and medical wipes due to its ability to eliminate some viruses, bacteria, and fungi. Another important use of ethanol is as a fuel in motor vehicles and eco-friendly fireplaces.
While pure ethanol consists of C2H5OH molecules, the classification of grain alcohol as a pure substance or a mixture seems to vary across different sources. Some sources classify grain alcohol, which is a form of ethanol, as a pure substance, while others consider it a mixture. This discrepancy may arise from the different interpretations or specific contexts related to the classification of substances.
Anger at an Alcoholic Mom: Normal Reaction?
You may want to see also

Mercury is an element with a constant composition
Pure substances are defined as materials with consistent compositions, while mixtures are combinations that retain individual properties. Mercury (Hg) is a chemical element that is composed of a single type of atom. It is a heavy, silvery-white liquid metal at room temperature and is commonly known as quicksilver. Mercury is the only metallic element that is liquid at standard temperature and pressure. It has a melting point of −38.83 °C and a boiling point of 356.73 °C, the lowest of any stable metal. Mercury is an extremely rare element in the Earth's crust, with an average crustal abundance by mass of only 0.08 parts per million (ppm). It is found either as a native metal (rare) or in cinnabar, metacinnabar, sphalerite, corderoite, livingstonite, and other minerals, with cinnabar (HgS) being the most common ore.
Mercury has no known biological role but is present in every living thing and is widespread in the environment. It is naturally occurring and found in rock in the Earth's crust, including in deposits of coal. Mercury easily forms alloys, called amalgams, with other metals such as gold, silver, and tin. It has been used in thermometers, barometers, manometers, and other devices, although concerns about its toxicity have led to a phase-out of mercury-containing instruments. Mercury dissolves many metals to form amalgams, but iron is an exception. Iron flasks have traditionally been used to transport mercury safely.
Mercury is a toxic substance that can pose serious health risks to humans and animals. It can cause mercury poisoning, which affects the nervous system, immune system, and kidneys. Methylmercury, a form of organic mercury, is particularly dangerous and can accumulate in the flesh of fish, leading to illness if consumed by people. Inorganic mercury compounds are still widely used in skin-lightening soaps and creams, and mercuric chloride is used in photography, disinfection, and wood preservation. Despite its toxicity, mercury has fascinated humans for millennia due to its unique properties as a heavy liquid metal.
In summary, mercury (Hg) is a pure substance or element with a constant composition. It has a consistent chemical formula, and its unique properties have led to various industrial and scientific applications. However, due to its toxicity, many uses of mercury are being phased out or replaced with safer alternatives.
Using 91% Alcohol in Ears: Is It Safe?
You may want to see also

Pure substances have a uniform composition
Pure substances are defined as materials with consistent compositions, while mixtures are combinations that retain individual properties.
Mercury is considered a pure substance. It is an element, denoted as Hg on the periodic table, and is composed of a single type of atom. Its properties, such as boiling point and density, remain constant.
Grain alcohol, or ethanol, is also classified as a pure substance. It consists of the same type of molecules (C2H5OH), making it a pure compound.
Other examples of pure substances include water (H2O), gold (Au), sugar (sucrose, C12H22O11), and oxygen (O2). These substances have consistent chemical formulas or are composed of a single type of atom.
On the other hand, a mixture is a combination of two or more substances, either elements or compounds, in any ratio. An example of a mixture is salt water, which is a solution of salt (NaCl) dissolved in water. Mixtures may exhibit the same properties as their constituent components.
How to Sneak Alcohol on a Plane Easily
You may want to see also

Mixtures are combinations of two or more substances
Pure substances are defined as materials with consistent compositions, while mixtures are combinations of two or more substances that retain individual properties. Mercury, for example, is a pure element with a constant composition and definite properties throughout. It is composed of a single type of atom (Hg) and does not consist of different substances mixed together.
On the other hand, grain alcohol, or ethanol, is considered a pure substance by some sources, as it consists of the same type of molecules (C2H5OH). However, other sources classify alcohol as a compound formed when two or more elements combine chemically in fixed proportions. Compounds are pure substances that exhibit different properties from their constituent elements. Alcohol is composed of carbon, hydrogen, and oxygen atoms, which are chemically bonded together. Beer, for instance, is considered a solution or a mixture of water, ethanol, and other substances.
In summary, while mercury is a pure substance, the classification of alcohol as a pure substance or a mixture depends on the context and specific type of alcohol being considered. Mixtures, in general, are combinations of two or more substances that retain the properties of their individual components.
Watered-Down Drinks: Iberostar Rose Hall Beach's Alcohol Mystery
You may want to see also

Pure substances are free from contamination
Pure substances are defined as materials with consistent compositions, while mixtures are combinations that retain individual properties. An element is a pure substance that has only one kind of particle, and these particles are atoms. Mercury (Hg) is an element composed of a single type of atom, and therefore it is a pure substance. Its properties, such as boiling point and density, remain constant. This means that mercury is free from contamination.
Grain alcohol, or ethanol, is also a pure substance. It consists of the same type of molecules (C2H5OH), making it a pure compound. A compound is a pure substance that contains two or more elements combined in a fixed proportion. The properties of a compound differ from the properties of the elements from which it is composed.
Gold is another example of a pure substance. It is a single type of atom (Au) and qualifies as a pure element. Other examples of pure substances include water, sugar, and oxygen.
Mixtures, on the other hand, are combinations of two or more substances that retain their individual properties. For example, salt water is a mixture of salt (NaCl) and water. The salt dissolves in the water, creating a homogeneous mixture. Gasoline is another example of a mixture.
Alcohol on Your Scalp: Good or Bad?
You may want to see also
Frequently asked questions
Mercury is a pure substance. It is an element composed of a single type of atom (Hg).
Grain alcohol, or ethanol, is a pure substance. It is a compound consisting of the same type of molecules (C2H5OH).
Pure substances have consistent compositions, while mixtures are combinations that retain individual properties. Pure substances can be elements or compounds. Elements consist of only one kind of particle or atom. Compounds are substances formed when two or more elements combine chemically in fixed proportions.
Saltwater is a mixture (a homogeneous mixture, to be precise) of salt (NaCl) and water.







