The conversion of esters into acids and alcohols is a fundamental concept in organic chemistry. Esters, which are formed through the combination of an organic acid (RCOOH) and an alcohol (ROH), can be converted back into their constituent parts through specific chemical reactions. This process involves the hydrolysis of esters in the presence of an alkali, resulting in the formation of carboxylic acids and alcohols. Additionally, the reduction of esters can yield primary or tertiary alcohols, depending on the choice of reagents. This transformation highlights the dynamic nature of chemical compounds and provides valuable insights into the behaviour of organic molecules.
| Characteristics | Values |
|---|---|
| Conversion of ester into acid | Hydrolysis of ester in the presence of alkali in an alcoholic solution under reflux, then neutralization by acid salt to get carboxylic acid |
| Hydrolysis of ester | Aqueous NaOH |
| Neutralization of reaction mixture | Aq.HCl |
| Conversion of ester into alcohol | Reduction by a strong reducing agent such as LiAlH4 to a primary alcohol |
| Conversion to a tertiary alcohol by reacting with two equivalents of Grignard or organolithium reagent |
What You'll Learn

Acidic reflux with dilute HCl/H2SO4
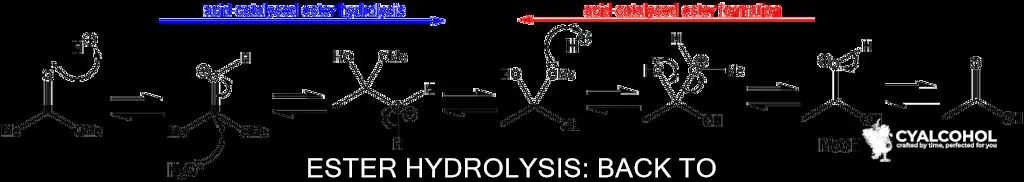
The Fischer esterification reaction involves the conversion of carboxylic acids to esters using an acid catalyst, such as H2SO4 (sulfuric acid) or TsOH (tosic acid). This reaction is reversible, and the forward reaction proceeds with the use of alcohol as a solvent, whereas the reverse reaction requires water as the solvent.
To convert an ester back into its constituent acid and alcohol, one can perform an acidic reflux with dilute HCl or H2SO4. Here is a step-by-step guide:
Preparation of the Mixture
Add 1 cm^3 of an alcohol, such as methanol, to a specimen tube containing dilute H2SO4. You can also use other alcohols, such as ethanol. Weigh out 0.2 g of a solid acid, such as benzoic acid or salicylic (2-hydroxybenzoic) acid, and add it to the tube.
Heating the Mixture
Place about 10 cm^3 of water into a beaker and gently heat it on a tripod and gauze until the water begins to boil. Carefully lower the tube containing the mixture into the beaker so that it stands upright. Stop heating and allow the tube to stand in the hot water for 1 minute. If the mixture in the tube boils, remove it from the water until the boiling stops, and then return it to the hot water.
Cooling and Mixing
After 1 minute, carefully remove the tube from the hot water and allow it to cool on a heat-resistant mat. When the mixture has cooled, pour it into a test tube partially filled with a sodium carbonate solution (approximately 0.5 M). This step may cause some effervescence. Mix the contents by pouring them back and forth between the specimen tube and the test tube, repeating this step if necessary.
Separation of Layers
A layer of ester will separate and float on top of the aqueous layer. The ester can be isolated from the mixture using a separating funnel, as catalysts are not consumed in this reaction. The organic layer containing the ester may also contain unreacted alcohol and carboxylic acid, particularly if they have lower solubility in water.
Purification
The ester is now separated from the aqueous layer, but it is important to purify it further. This is because the organic layer may still contain unreacted alcohol and carboxylic acid, which can affect the purity of the final product.
Conversion to Acid and Alcohol
The ester can be hydrolyzed using water to convert it back into its constituent acid and alcohol. This reaction is the reverse of the Fischer esterification and can be facilitated by using water as the solvent. The specific conditions and reagents required may vary depending on the specific ester and the desired yield and purity.
Spraying Alcohol on Surgical Masks: Is It Safe?
You may want to see also

Reflux in alcohol with a dilute first group alkali
To convert an ester back into an acid and alcohol, one method involves refluxing the ester in alcohol with a dilute first group alkali. This process takes advantage of the reversibility of the Fischer esterification reaction, where the ester is hydrolysed back into its constituent acid and alcohol components.
The first step is to identify the type of alcohol involved in the reaction, as this will determine the products formed during the reflux process. There are three types of alcohols: primary, secondary, and tertiary. Primary alcohols are the easiest to oxidize, as they have a hydrogen atom attached to the carbon atom bonded to the hydroxyl group (-OH). Tertiary alcohols, on the other hand, cannot be oxidized under typical conditions because they lack this hydrogen atom.
When refluxing a primary alcohol, the alcohol is first oxidized to an aldehyde, which can be identified by a colour change from orange to green in the presence of potassium dichromate(VI). If the reaction is allowed to proceed further, the aldehyde undergoes complete oxidation to form a carboxylic acid. This second step can be prevented by using an excess of alcohol, which ensures there is not enough oxidizing agent for the second stage, and by distilling off the aldehyde as soon as it is formed.
For secondary alcohols, the oxidation typically leads to the formation of ketones rather than proceeding to the carboxylic acid stage. Tertiary alcohols are unique in that they can only be oxidized by combustion and do not exhibit a colour change when mixed with potassium dichromate(VII).
It is important to note that the reflux process should be carefully monitored to control the extent of oxidation. This can be achieved by using mild oxidizing agents for partial oxidation to aldehydes and stronger agents for complete oxidation to carboxylic acids.
Converting Alcohol: KG to Gal Formula Simplified
You may want to see also

Hydrolysis of ester with aqueous NaOH
The conversion of an ester back into an acid and an alcohol is called hydrolysis. This process is also known as saponification, as the carboxylate salts initially formed through hydrolysis are often used as soaps.
The first step in saponification is the nucleophilic addition of the hydroxide ion to the carbonyl carbon of the ester to form a tetrahedral intermediate. This is followed by the elimination of the alkoxide (RO–) from the tetrahedral intermediate to give a carboxylic acid. This two-step addition-elimination process is an example of nucleophilic acyl substitution.
When performing hydrolysis of an ester with aqueous NaOH, it is important to note that the reaction is irreversible under basic conditions. This means that obtaining the ester back is highly unfavourable. The first reaction that occurs under basic conditions is an acid-base reaction between the alkoxide and the carboxylic acid, forming a carboxylate salt. Alcohols are much less acidic than carboxylic acids, making this an irreversible reaction.
To obtain the neutral carboxylic acid, one can add a strong acid to the aqueous solution of carboxylate until the carboxylic acid precipitates out, and then perform an extraction with an organic solvent.
Lithium cations have been observed to accelerate the hydrolysis of esters with hydroxides (KOH, NaOH, LiOH) in a water/tetrahydrofuran (THF) two-phase system.
FAS and Child Endangerment: Pennsylvania's Stance
You may want to see also

Reduction by a strong reducing agent
Esters can be converted to alcohols by two types of reduction reactions. One of these is reduction by a strong reducing agent, such as lithium aluminium hydride (LiAlH4). This method produces a primary alcohol.
The reduction of an ester to an alcohol requires two hydride additions to the carbonyl group, which is why an excess of LiAlH4 is used. After the first hydride addition, a tetrahedral intermediate is formed. This contains a leaving group, which is then kicked out, reforming the carbonyl group. This newly formed carbonyl group is an aldehyde and is more reactive than the ester. As a result, it is attacked once more by LiAlH4.
This reduction can also be achieved with DIBAL-H, a weaker reducing agent than LiAlH4. The reaction with DIBAL-H is usually carried out at -78°C to prevent reaction with the aldehyde product.
The mechanism of action of hydride reductions on esters is analogous to the hydride reduction of carboxylic acids. Nucleophilic acyl substitution replaces the –OR leaving group in the ester with a hydride nucleophile to form an aldehyde intermediate. Because aldehydes are more reactive than esters, they rapidly undergo a second nucleophilic hydride addition to form a tetrahedral alkoxide intermediate. An acid work-up protonates the alkoxide to create a 1o alcohol.
Another strong reducing agent that can be used to convert esters to alcohols is lithium tri-tert-butoxyaluminium hydride (LiAlH(Ot-Bu)3).
Bagging Alcohol: New York's Unique State Law
You may want to see also

Conversion to a tertiary alcohol
Esters can be converted to alcohols through two types of reduction reactions. The first reaction involves the use of a strong reducing agent, such as LiAlH4, to form a primary alcohol. The second reaction involves converting the ester into a tertiary alcohol. This conversion requires reacting the ester with two equivalents of a Grignard reagent or an organolithium reagent.
The reduction of an ester to a tertiary alcohol involves two hydride additions to the carbonyl group. An excess of LiAlH4 is typically used due to the presence of a leaving group in the tetrahedral intermediate formed after the initial hydride addition. This leaving group is expelled, resulting in the reformation of the carbonyl group. Notably, the newly formed carbonyl group is more reactive than the ester, making it susceptible to a second attack by LiAlH4.
The conversion of an ester to a tertiary alcohol through the use of a Grignard reagent involves two steps. The first step entails a nucleophilic attack by the Grignard reagent, leading to the formation of a C-C bond and a shift in the electrons of the π bond towards oxygen. The product of this initial addition-elimination reaction is a ketone, which is then converted into a tertiary alcohol in the second step.
Similarly, organolithium reagents can also effectively convert an ester into a tertiary alcohol. This process is comparable to the mechanism observed with Grignard reagents, where the first addition-elimination reaction yields a ketone that subsequently undergoes conversion into a tertiary alcohol.
These reactions provide valuable tools for synthetic organic chemists, enabling the transformation of esters into tertiary alcohols with specific structural and reactivity profiles.
Alcohol and Stimulants: Beauty's Dark Side
You may want to see also







