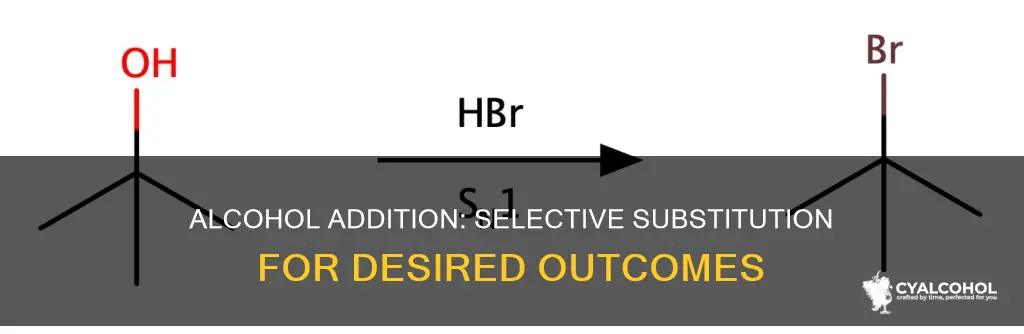
There are several methods for adding an alcohol to the least substituted side of an alkene. One method is hydroboration, which involves the addition of borane (BH3) in a tetrahydrofuran solvent (THF) to the alkene, followed by the addition of hydrogen peroxide (H2O2) and sodium hydroxide (NaOH). This results in the formation of an anti-Markovnikov alcohol, where the alcohol is added to the least substituted carbon. Another method is oxymercuration-demercuration, which converts alkenes into Markovnikov-product alcohols. During this process, the hydroxyl group bound to the more substituted carbon guides the hydrogen towards the less substituted carbon, eventually forming a Markovnikov product.
| Characteristics | Values |
|---|---|
| Process | Hydroboration |
| Reagents | Borane (BH3), tetrahydrofuran solvent (THF), hydrogen peroxide (H2O2), sodium hydroxide (NaOH) |
| Mechanism | Cyclic transition state, borane adds to the least substituted side of the double bond |
| Reaction | Anti-Markovnikov |
| Alternative Processes | Oxymercuration-demercuration, acid-catalyzed hydration |
What You'll Learn

Hydroxyl addition to the most substituted carbon
The nucleophile then attacks the positive charge formed on the most substituted carbon connected to the double bond, as it is attracted to the positive charge. Water acts as the nucleophile in this reaction, and the removal of a hydrogen atom from the water molecule results in the attachment of the alcohol to the most substituted carbon. This process follows Markovnikov's rule, which states that the halogen or nucleophile tends to attach to the carbon of the alkene with the fewest hydrogen atoms.
The oxymercuration-demercuration reaction is another method to achieve hydroxyl addition to the most substituted carbon. This reaction converts alkenes into Markovnikov-product alcohols. It involves the use of mercuric acetate, Hg(OAc)2, and water, followed by the addition of sodium borohydride, NaBH4. However, it is important to note that this reaction requires the use of mercury, which is highly toxic.
Another technique to achieve hydroxyl addition to the least substituted carbon is through hydroboration-oxidation. This method involves adding borane (BH3) to the alkene, followed by the addition of hydrogen peroxide (H2O2) and sodium hydroxide (NaOH). The boron atom adds to the least substituted carbon, while the hydrogen atom attaches to the more substituted carbon. This reaction proceeds in an anti-Markovnikov manner, opposite to the usual Markovnikov addition.
It is important to consider regiochemistry, which deals with the position of the substituent on the product. In the case of electrophilic hydration, Markovnikov's rule is applicable. Stereochemistry is also relevant, as it addresses how the substituent bonds to the product directionally. These factors influence the outcome of hydroxyl addition reactions and provide insights into the positional specificity of substituents.
Alcohol Strength for First-Aid: What's the Minimum?
You may want to see also

Anti-Markovnikov's rule
The Anti-Markovnikov rule is an exception to the general rule of regioselective reactions in organic chemistry, known as Markovnikov's rule. Markovnikov's rule states that when a protic acid HX or other polar reagent is added to an asymmetric alkene, the acid hydrogen (H) group attaches to the carbon with more hydrogen substituents, while the halide (X) group attaches to the carbon with more alkyl substituents. In other words, the nucleophile will add to the more substituted pi-bound carbon, leaving hydrogen to add to the less substituted carbon.
The Anti-Markovnikov rule, on the other hand, defines regiochemistry where the substituent is attached to a less substituted carbon instead of the more substituted carbon. This is because a substituted carbocation allows for more hyperconjugation and induction, resulting in a more stable carbocation. When a protic acid HX or other polar reagent is added to an asymmetric alkene in this scenario, the acid hydrogen (H) group attaches to the carbon with more alkyl substituents, while the halide (X) group attaches to the carbon with more hydrogen substituents.
Anti-Markovnikov behaviour is observed in several chemical reactions, including the hydration of phenylacetylene by auric catalysis, which yields acetophenone. With a special ruthenium catalyst, the reaction produces the other regioisomer, 2-phenylacetaldehyde. Anti-Markovnikov behaviour is also observed in certain rearrangement reactions, such as titanium(IV) chloride-catalyzed formal nucleophilic substitution at enantiopure 1, resulting in the formation of two products, 2a and 2b.
The Anti-Markovnikov rule is particularly applicable in free radical addition reactions, where the regioselectivity of the mechanisms is not predicted by Markovnikov's rule. An example of this is when HBr is added to unsymmetrical alkenes in the presence of peroxide, forming 1-bromopropane instead of 2-bromopropane, which would be expected according to Markovnikov's rule. This reaction is known as the Kharash effect, named after M. S. Kharash, who first observed it.
In summary, the Anti-Markovnikov rule describes a regioselective reaction where the substituent attaches to the less substituted carbon, contrary to Markovnikov's rule, which states that the substituent adds to the more substituted carbon. This rule is observed in specific chemical reactions, particularly free radical addition reactions, and results in unique product distributions.
Alcohol Abuse: When Does Binging Become a Problem?
You may want to see also

Oxymercuration-demercuration
The oxymercuration-demercuration reaction follows Markovnikov's Regioselectivity, with the OH group attached to the most substituted carbon and the H attached to the least substituted carbon. This is the opposite of the anti-Markovnikov product, where the OH group is attached to the least substituted carbon. To make the anti-Markovnikov product, hydroboration is used. Borane (BH3) is added to the alkene in a tetrahydrofuran solvent (THF), followed by the addition of hydrogen peroxide (H2O2) and sodium hydroxide (NaOH). This makes the anti-Markovnikov alcohol.
The oxymercuration step is stereoselective, while the demercuration step is not. The oxymercuration-demercuration mechanism is an electrophilic addition reaction because there are no carbocation rearrangements due to the stabilization of the reactive intermediate. This intermediate is an organomercury compound, which is rarely isolated. Instead, demercuration is performed with sodium borohydride (NaBH4), which breaks the C-Hg bond and forms a new C-H bond. This step can also be carried out with sodium borodeuterohydride (NaBD4) to form the deuterated product.
Overall, the oxymercuration-demercuration reaction is a useful method for converting alkenes into alcohols, as it does not require strong acids and avoids carbocation rearrangements.
Alcohol Sharing: Legal When Parents Are Involved?
You may want to see also

Hydroboration-oxidation
The first step of hydroboration-oxidation involves the hydroboration of an alkene (also known as an olefin) with a borane (BH3). The borane acts as a Lewis acid by accepting two electrons in its empty p orbital from an alkene that is electron-rich. This allows boron to have an electron octet. The boron adds to the less substituted carbon of the alkene, which then places the hydrogen on the more substituted carbon. Both boron and hydrogen add simultaneously on the same face of the double bond (syn addition).
The second step of the mechanism involves the oxidation of the organoborane product from the first step. This is done by treating the organoborane with an oxidant like hydrogen peroxide (H2O2) to obtain an alcohol. The key step of the oxidation reaction is the shifting of a pair of electrons in the C-B bond to form a new C-O bond, with the breakage of the relatively weak O-O bond. This step proceeds with syn stereochemistry, where the alcohol and hydrogen add to the same face of the double bond.
Overall, the hydroboration-oxidation reaction is a useful and important method for forming alcohols from alkenes. It provides a stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration-reduction process.
Winterizing Boats: Propylene Glycol and Ethyl Alcohol Safety
You may want to see also

Grignard reagent reaction with epoxides
Grignard reagents are carbon-based nucleophiles that can be combined with various electrophilic carbon species to form new carbon-carbon bonds. This class of reactions is very useful as carbon-carbon bonds constitute the "backbone" of molecules in organic chemistry.
Grignard reagents react with epoxides, which are 3-membered cyclic ethers that possess considerable ring strain. This ring strain makes epoxides "spring-loaded" toward attack by nucleophiles, resulting in the formation of a new bond to carbon and the opening of the ring.
The reaction of Grignard reagents with epoxides follows an SN2-like mechanism, with the nucleophile attacking the least substituted carbon of the epoxide. This attack occurs from the backside, leading to an inversion of configuration at the attacked carbon. For example, if the epoxide has a substituent pointing forward (on a wedge), the new group will be positioned backward (on a dash) after the Grignard attack.
To synthesize a specific secondary alcohol using this reaction, one can work backward using retrosynthetic analysis. The carbon bearing the hydroxyl group in the target molecule was originally part of the epoxide, and the new carbon-carbon bond formed comes from the Grignard reagent. By identifying the bond formed, one can break the molecule into the corresponding epoxide and Grignard reagent that would react to give the desired product.
For instance, if one aims to form a four-carbon chain extension, butylmagnesium bromide can be used as the Grignard reagent and reacted with an appropriately substituted epoxide. It is important to carefully number the carbons on both the starting materials and products to avoid miscounting carbon atoms.
Overall, the Grignard reaction with epoxides is a powerful tool in synthesis, allowing for the formation of carbon-carbon bonds, which is a key goal in many synthetic strategies. However, it is important to carefully analyze regioselectivity and stereochemistry, as not every combination will work.
Alcohol Cessation: Gradual Weaning or Cold Turkey?
You may want to see also
Frequently asked questions
The Markovnikov rule describes where the substituent bonds onto the product. In the context of adding an alcohol to the least substituted side, it refers to the addition of a proton to the less substituted carbon in the double bond, followed by the addition of the -OH group to the more substituted carbon.
The anti-Markovnikov rule refers to the addition of the alcohol (OH group) to the least substituted carbon in the double bond. This results in the formation of an anti-Markovnikov product.
To add an alcohol to the least substituted side, you can use the hydroboration-oxidation method. This involves adding borane (BH3) in a tetrahydrofuran solvent (THF) to the alkene, followed by the addition of hydrogen peroxide (H2O2) and sodium hydroxide (NaOH). The borane adds to the least substituted side of the double bond, resulting in the formation of an alkyl borane.