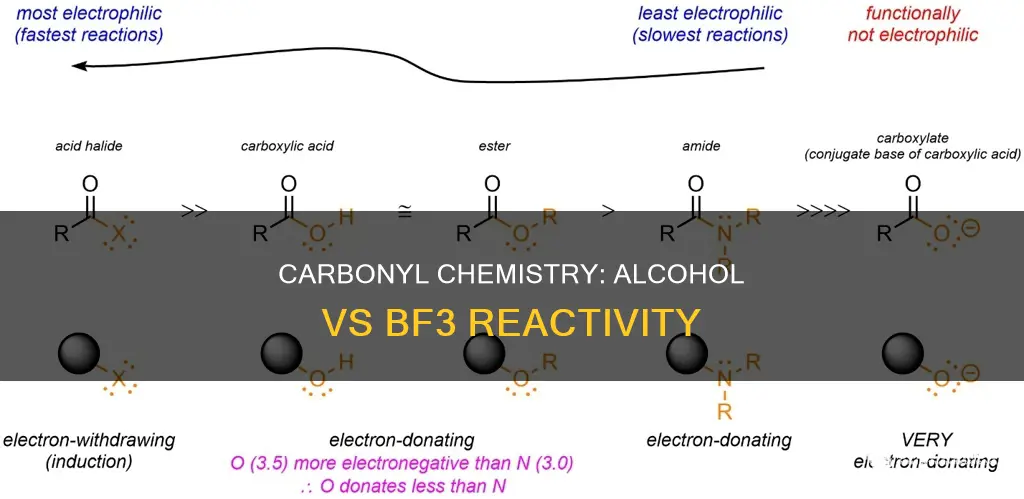
The reactivity of alcohols and carbonyls is a topic of interest in organic chemistry. Alcohols (R-OH) can add to carbonyl groups, which are characterized by a carbon-oxygen double bond (C=O). Aldehydes and ketones, two functional groups containing a carbonyl group, can undergo nucleophilic addition reactions, reductions, and reactions with ammonia or amines to form derivatives. Alcohols, on the other hand, primarily react via their hydroxyl (-OH) groups and are involved in acid-base reactions and dehydration reactions. Lewis acids, such as BF3, can also be used to facilitate specific reactions with alcohols, such as SN1 and SN2 reactions. This comparison of reactivity between alcohols and carbonyl groups provides insights into their distinct chemical behaviors and their potential applications in various organic synthesis reactions.
What You'll Learn
- Alcohols are less reactive than aldehydes due to their molecular structure
- Aldehydes have a highly polarised carbonyl group, making them more reactive
- Lewis acids like BF3 can be used to make leaving groups out of alcohols for SN1 reactions
- Aldehydes are more prone to nucleophilic attacks due to their electron-deficient carbonyl group
- Alcohols primarily undergo acid-base reactions, while aldehydes are more versatile

Alcohols are less reactive than aldehydes due to their molecular structure
Alcohols are generally less reactive than aldehydes due to differences in their molecular structure. Alcohols possess a hydroxyl group (-OH) that is polar but less reactive than the carbonyl group (C=O) in aldehydes. The carbonyl group in aldehydes is highly polarised, with a partially positive carbon atom that is electron-deficient. This electron-deficient carbon makes aldehydes more prone to nucleophilic attacks, where an electron-rich species can attack the positively polarised carbon atom.
The unique molecular structure of aldehydes, with their carbonyl group at the end of the carbon chain, makes them more reactive in a variety of reactions, including nucleophilic addition and oxidation. On the other hand, alcohols primarily engage in acid-base reactions and dehydration reactions, which are limited in comparison. For example, acetaldehyde (an aldehyde) can easily react with nucleophiles like sodium borohydride to form alcohols, whereas ethanol (an alcohol) requires stronger conditions for reactions like dehydration.
The reactivity of aldehydes and alcohols can be observed in their reactions with each other. When aldehydes react with alcohols, they produce hemiacetals or acetals, depending on the conditions. The nucleophilic substitution of an OH group for the double bond of the carbonyl group forms hemiacetals, while mixing aldehydes and alcohols with hydrochloric acid produces acetals.
Furthermore, aldehydes and ketones can be reduced to alcohols through reactions with metal hydrides like lithium aluminium hydride and sodium borohydride. These reductions decrease the oxidation state of the carbonyl carbon, making it less reactive. Overall, the molecular structure of aldehydes, with their polarised carbonyl group, makes them more reactive than alcohols, which have a less reactive hydroxyl group.
Alcohol in Saudi Arabia: A New Era?
You may want to see also

Aldehydes have a highly polarised carbonyl group, making them more reactive
The reactivity of aldehydes and ketones is due to the presence of the carbonyl group, which consists of a carbon-oxygen double bond (C=O). This functional group is highly polarised, with the carbon atom having a partial positive charge and the oxygen atom carrying a partial negative charge. This polarity arises from the difference in electronegativity between carbon and oxygen. The aldehyde carbonyl group is more electron-deficient compared to ketones because aldehydes have only one electron-donating group (usually hydrogen), while ketones have two (usually organic groups).
The electron-deficient nature of the carbonyl group in aldehydes makes them highly reactive. Specifically, the carbonyl carbon is susceptible to nucleophilic attacks by electron-rich species. This is because the partially positive carbon atom attracts electron-rich nucleophiles, which can then react with the carbonyl group. This reactivity allows aldehydes to participate in a wide range of nucleophilic addition reactions, including reactions with nucleophiles like sodium borohydride to form alcohols.
In contrast, alcohols possess hydroxyl (-OH) groups, which are polar but less reactive compared to the electron-deficient carbonyl group. Alcohols primarily undergo reactions involving their hydroxyl groups, such as acid-base reactions or dehydration reactions. For example, ethanol (an alcohol) requires stronger conditions for dehydration reactions compared to aldehydes.
The unique molecular structure of aldehydes, with their highly polarised carbonyl group, makes them more reactive than alcohols. This higher reactivity leads to a broader range of chemical reactions, including nucleophilic addition and oxidation reactions.
Furthermore, aldehydes are less hindered than ketones due to their smaller hydrogen atom compared to the bulkier organic groups in ketones. This lower steric hindrance in aldehydes facilitates reactions by allowing easier access to the reactive carbonyl group. Therefore, the combination of a highly polarised carbonyl group and lower steric hindrance makes aldehydes more reactive than alcohols.
Alcohol in Cancun: All-Inclusive Resort Drinks
You may want to see also

Lewis acids like BF3 can be used to make leaving groups out of alcohols for SN1 reactions
Alcohols are poor nucleophiles and substrates for SN1 reactions in their neutral form. This is due to the hydroxide group (HO–) being a poor leaving group. However, Lewis acids like BF3 can be used to make good leaving groups out of alcohols for SN1 reactions. This is done by protonating the hydroxyl group R–OH into R–OH2(+), which is a
The SN1 reaction generally proceeds in two steps: the first is the breaking of the C–LG bond on the substrate to form an intermediate carbocation, and the second is the fast addition of a nucleophile to the carbocation. The rate-limiting step is the loss of the leaving group to give a carbocation. Polar protic solvents, such as water, alcohols, or carboxylic acids, can stabilize the resulting carbocation through hydrogen bonding or other dipolar interactions.
In the context of teaching a class, one user confirms that BF3 can be used to cleave tBu ethers, and therefore, it should work for making leaving groups out of alcohols for SN1 reactions. However, they note that in actual practice, HBr would be used instead because it is cheaper and easier to handle.
Additionally, it is important to consider the sterics of the reaction. The methyl groups surrounding the oxygen in the alcohol may make it less accessible for interaction with BF3 due to its relatively larger size compared to HBr. As a result, the reaction may proceed more slowly or not at all.
Alcohol Consumption: Is It Safe for 18-Year-Olds?
You may want to see also

Aldehydes are more prone to nucleophilic attacks due to their electron-deficient carbonyl group
Aldehydes and ketones are classified as carbonyl compounds, which contain a carbonyl group with a carbon-oxygen double bond. This bond is polar due to the difference in electronegativity between oxygen and carbon, resulting in a partial positive charge on the carbonyl carbon. This carbon is susceptible to nucleophilic attacks, where a nucleophile, or electron donor, interacts with the partially positive carbon.
Aldehydes are more prone to these nucleophilic attacks compared to ketones due to several factors. Firstly, aldehydes have a greater polarization of the carbonyl bond, making the carbonyl carbon more electrophilic and attractive to nucleophiles. This is because the primary carbocation formed in the polarizing resonance structure of an aldehyde is less stable and, therefore, more reactive than the secondary carbocation found in ketones. The presence of an extra alkyl group in ketones contributes to the stability of their carbocations.
Additionally, aldehydes are less hindered at the carbonyl carbon than ketones. Ketones have two bulkier alkyl groups attached, while aldehydes always have at least one hydrogen atom attached. Since alkyl groups are electron-donating, they partially offset the positive charge on the carbonyl carbon in ketones, making aldehydes relatively more susceptible to nucleophilic attack.
The reactivity of aldehydes and ketones can be influenced by their substituents. For example, aromatic rings act as electron-donating groups, reducing the electrophilicity of the carbonyl group and decreasing their reactivity towards nucleophiles.
In terms of reactivity with other compounds, alcohols are considered weak nucleophiles and require an acid catalyst to activate the carbonyl group of aldehydes or ketones before they can undergo nucleophilic attack. While alcohols can react with both aldehydes and ketones, the specific reactivity between alcohols and ketones versus PCl5 is a separate topic with its own considerations.
Young Adults: Alcohol Dependency Risk Factors
You may want to see also

Alcohols primarily undergo acid-base reactions, while aldehydes are more versatile
Alcohols are weak bases that can act as acids or bases. They can undergo acid-base reactions, which are key to understanding their reactivity. In these reactions, a protonated alcohol becomes a better electrophile, with the conjugate acid acting as a better leaving group. The hydroxyl groups in R-OH are poor nucleophiles due to their neutral nature and the tight hold on the electron pair by the oxygen atom. However, if a proton is removed by adding a base, an alkoxide ion (RO-) is formed, which has a higher electron density and is a superior nucleophile. This alkoxide ion is also a strong base.
Alcohols can be categorised as primary, secondary, or tertiary, depending on the number of carbons attached to the carbon atom bearing the hydroxyl group. They have high boiling points due to hydrogen bonding. Alcohols can be oxidised to aldehydes and acids by dehydrogenases, a crucial reaction in alcohol metabolism. While they are not readily oxidised to free radicals by one electron, they will react with hydroxyl radicals to form hydroxymethyl radicals.
Aldehydes, on the other hand, do not display hydrogen bonding. However, the carbonyl group in aldehydes can act as a hydrogen bond acceptor with a hydrogen bond donor like water. This makes aldehydes less soluble in water compared to alcohols.
While alcohols primarily undergo acid-base reactions, aldehydes offer more versatility in their reactivity. Aldehydes can act as electrophiles and alkylate nucleophiles. Additionally, aldehydes can exhibit acute irritant effects on humans, with acrolein being the most potent among commonly studied aldehydes.
In terms of reactivity with PCl5, alcohol is more reactive than ketone. This is because it is challenging for the ketone to donate its electron pair to PCl5 due to the strong C=O bond.
In summary, while alcohols are weak bases that primarily undergo acid-base reactions, aldehydes offer more versatility in terms of reactivity and can participate in a wider range of reactions.
Does Barbican Contain Alcohol?
You may want to see also







