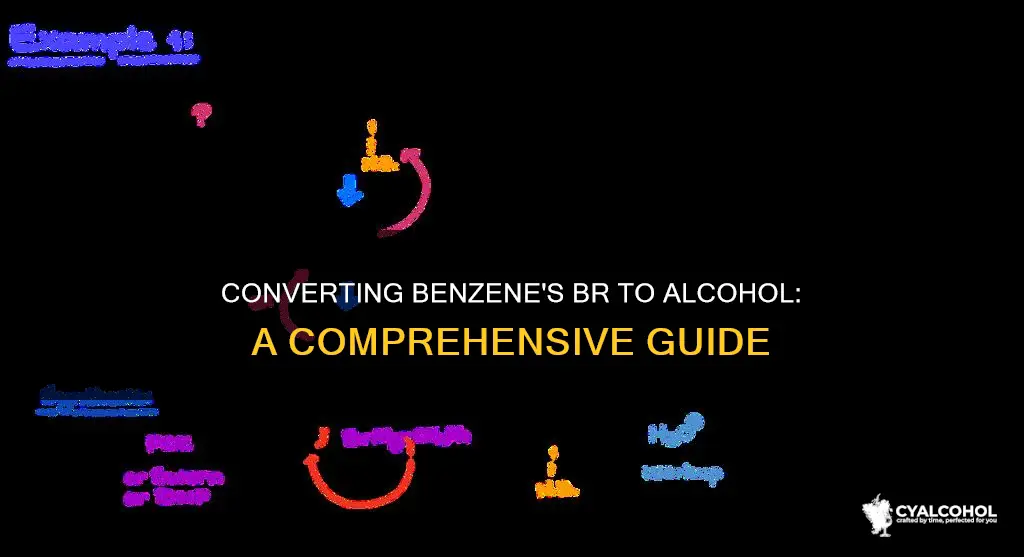
Benzene is a very stable organic compound. Converting benzene to benzyl alcohol, an organic compound in which a hydroxyl group is attached to a benzene ring, cannot be done in one step. Instead, it involves several processes and the formation of different compounds. One method involves first reacting benzene with ${{C}}{{{H}}_3}{{Cl}}} in the presence of aluminium chloride as a catalyst. This results in the substitution of the methyl group in the benzene ring, forming toluene. The toluene is then oxidized with a potassium permanganate solution to give benzoic acid. Finally, the benzoic acid is reduced using a suitable reducing agent like ${{LiAl}}{{{H}}_4}$ to yield benzyl alcohol. Another method involves chlorinating toluene and then heating it with aqueous sodium hydroxide or potassium hydroxide to produce benzyl alcohol.
| Characteristics | Values |
|---|---|
| Number of steps | Multiple |
| First step | Friedel-Crafts alkylation reaction of benzene |
| First step reaction | Benzene with \({{CH}_3}{{Cl}}\) in the presence of aluminium chloride as a catalyst |
| Second step | Oxidation of toluene with potassium permanganate solution to give benzoic acid |
| Third step | Oxidation of the methyl group outside the benzene ring to form a carboxylic acid |
| Third step medium | Basic |
| Third step addition | Acidic hydrogen ions |
| Third step oxidizing agent | \({{KMn}}{{{O}}_4}\) |
| Fourth step | Reduction of benzoic acid to benzyl alcohol |
| Reducing agent | \({{LiAL}}{{{H}}_4}\) |
| Alternative first step | Chlorination of toluene |
| Alternative second step | Heat chlorinated toluene with aqueous sodium hydroxide or potassium hydroxide |
What You'll Learn

Friedel-Crafts alkylation reaction of benzene
The conversion of Br on benzene to an alcohol involves several processes and the formation of different compounds. One of the initial steps is the Friedel-Crafts alkylation reaction of benzene. This is an electrophilic substitution reaction where an alkyl group is substituted into a benzene ring.
The Friedel-Crafts alkylation reaction of benzene involves the following steps:
Step 1: Generation of a Methyl Carbocation
The reaction begins with the generation of a methyl carbocation from methylbromide. This carbocation reacts with the pi electron system of the benzene ring.
Step 2: Formation of a Non-Aromatic Carbocation
The carbocation formed in Step 1 then reacts with the pi electron system of the benzene ring, forming a non-aromatic carbocation.
Step 3: Delocalization of the Positive Charge
The positive charge on the carbocation formed in Step 2 is delocalized throughout the molecule.
Step 4: Restoration of Aromaticity
The aromaticity of the molecule is restored through the loss of a proton from the atom to which the methyl group has bonded. This results in the substitution of a hydrogen on the benzene ring with a methyl group, forming methylbenzene (also known as toluene).
Overall Reaction
The overall reaction can be summarized as follows: Benzene is treated with a chloroalkane (such as chloromethane or chloroethane) in the presence of aluminum chloride as a catalyst. This reaction substitutes a hydrogen on the benzene ring with a methyl group, forming toluene.
After the Friedel-Crafts alkylation reaction, the toluene is further oxidized to form benzyl alcohol. This involves oxidizing the methyl group outside the benzene ring to form a carboxylic acid, followed by the addition of acidic hydrogen ions. Potassium permanganate ($KMnO_4$) is commonly used as the oxidizing agent for benzene oxidation. Finally, the benzoic acid is reduced to obtain benzyl alcohol using a suitable reducing agent like $LiAlH_4$.
Alcohol Overdose: What You Should Do to Help
You may want to see also

Oxidation of toluene to benzoic acid
The conversion of benzene to benzyl alcohol involves several processes and the formation of different compounds. One of the intermediate compounds formed during this process is benzoic acid.
Friedel-Crafts Alkylation Reaction of Benzene
The first step is to react benzene with $CH_3Cl$ in the presence of aluminium chloride as a catalyst. This results in the substitution of the methyl group in the benzene ring, forming toluene.
The toluene formed in the previous step is then oxidized to benzoic acid. This can be achieved using a potassium permanganate solution or by using manganese oxides as a catalyst with molecular oxygen as the oxidant. The use of manganese oxides has the advantage of being easily prepared, simple to use, and recyclable.
Reduction of Benzoic Acid to Benzyl Alcohol
To obtain benzyl alcohol, the benzoic acid is reduced using a suitable reducing agent like $LiAlH_4$.
Alternatively, toluene can be converted to benzyl alcohol in two steps. First, toluene is chlorinated, forming benzyl chloride. Then, benzyl chloride reacts with sodium hydroxide or potassium hydroxide to yield benzyl alcohol.
Soaking Male Masturbators: Safe with Alcohol?
You may want to see also

Reduction of benzoic acid to benzyl alcohol
The conversion of benzene to benzyl alcohol is a multi-step process that cannot be accomplished in a single step. Benzyl alcohol is an organic compound in which a hydroxyl group is attached to a $- {CH_2}$ group, which is attached to a benzene ring.
The first step in the process is the Friedel-Crafts alkylation reaction of benzene. This is achieved by reacting benzene with $CH_3Cl$ in the presence of an aluminium chloride catalyst. This reaction substitutes a methyl group into the benzene ring, resulting in toluene.
The next step is to oxidise the toluene with a potassium permanganate solution, $KMnO_4_, to form benzoic acid. The methyl group outside the benzene ring gets oxidised to form a carboxylic acid. This reaction occurs in a basic medium, followed by the addition of acidic hydrogen ions.
The benzoic acid must then be reduced to obtain benzyl alcohol. A suitable reducing agent, such as $LiAlH_4$, is used for this step.
Alternatively, toluene can be converted to benzyl alcohol through a different set of reactions. Toluene is first chlorinated and then heated with an aqueous solution of sodium hydroxide or potassium hydroxide. This process forms benzyl chloride, which then reacts with sodium hydroxide or potassium hydroxide to yield benzyl alcohol.
The Art of Shaving: Alcohol-Free After-Shave?
You may want to see also

Conversion of toluene to benzyl chloride
The conversion of toluene to benzyl chloride involves a series of chemical reactions.
Firstly, toluene (C6H5CH3) is treated with alkaline potassium permanganate (KMnO4). This oxidises the methyl group (–CH3) of toluene to a carboxylic acid (–COOH), forming benzoic acid (C6H5COOH).
The second step involves treating benzoic acid with lithium aluminum hydride (LiAlH4), a strong reducing agent. This converts the carboxylic acid group (–COOH) into an alcohol (–CH2OH), resulting in benzyl alcohol (C6H5CH2OH).
Finally, benzyl alcohol undergoes a nucleophilic substitution reaction where the hydroxyl group (–OH) is replaced by a chlorine atom (Cl) by treating it with phosphorus pentachloride (PCl5). This forms the final product, benzyl chloride.
An alternative method to convert toluene to benzyl chloride involves chlorinating toluene in the presence of light. This directly forms benzyl chloride, which can then be reacted with aqueous sodium hydroxide or potassium hydroxide to form benzyl alcohol.
It is important to note that the conversion of benzene to benzyl alcohol cannot be achieved in a single step and requires multiple processes and the formation of different compounds.
Alcoholism: Internal or External Literary Conflict?
You may want to see also

Conversion of benzyl chloride to benzyl alcohol
Converting benzyl chloride to benzyl alcohol involves a few steps and reactions. Benzyl alcohol is an organic compound in which a hydroxyl group is attached to a $- {C{H_2}}$ group, which is attached to the benzene ring.
First, we need to identify the reactants and products. Benzyl chloride ($C6H5CH2Cl$) is the starting material, and benzyl alcohol ($C6H5CH2OH$) is the desired product.
Next, we choose the reagent. To convert benzyl chloride to benzyl alcohol, we need a suitable reagent. In this case, an aqueous solution of potassium hydroxide (KOH) is used.
Now, we can understand the reaction mechanism. The reaction involves a nucleophilic substitution. The hydroxide ion (OH-) from the aqueous KOH acts as a nucleophile and attacks the carbon atom bonded to the chlorine atom in benzyl chloride.
The reaction is performed by mixing benzyl chloride with the aqueous solution of KOH. The hydroxide ion replaces the chlorine atom, resulting in the formation of benzyl alcohol.
The balanced chemical equation for the reaction is:
$$ \text{C}6\text{H}5\text{CH}2\text{Cl} + \text{KOH (aq)} \rightarrow \text{C}6\text{H}5\text{CH}2\text{OH} + \text{KCl} $$
Another method to convert benzyl chloride to benzyl alcohol involves first converting benzene to toluene through a Friedel-Crafts alkylation reaction. Toluene is then oxidized to benzoic acid, which is then reduced to benzyl alcohol. This process is lengthier and involves more steps than the previous method.
Alcohol on Head Wounds: Is It Safe?
You may want to see also