Benzene is a highly stable compound with unusual reactivity. It is challenging to hydrogenate and does not react with typical nucleophiles. However, benzene exhibits electrophilic substitution reactions, which can be leveraged to introduce functional groups. One example is the reaction of benzene with ketones or aldehydes in the presence of acid catalysts to form benzyl alcohol. This reaction involves protonating the oxygen atom of the carbonyl group, generating a carbocation that can undergo an electrophilic attack on the benzene ring. While this reaction is possible, it may not be commonly employed due to the activation energy required for a simple aldehyde to initiate the process. Additionally, the electronegativity of the benzene carbon atom, influenced by its sp^2 hybridization, further complicates the generation of the desired carbocation. Nevertheless, understanding benzene's reactivity and exploring alternative reagents, such as phenyl lithium or phenyl magnesium bromide, can provide alternative synthetic routes to incorporate secondary alcohols into the benzene ring.
| Characteristics | Values |
|---|---|
| Synthesis of secondary alcohols | Using Grignard reagent with aldehydes |
| Reducing reagents | Sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) |
| Benzene reaction | Acid-catalysed reaction with ketone to form benzyl alcohol |
| Benzene properties | Unusually difficult to hydrogenate, requires high temperatures, pressures, and reaction times |
| Benzene substitution | Friedel-Crafts alkylation, acylation, chlorination, bromination, nitration, and sulfonation |
| Benzene stability | Higher than expected for theoretical "cyclohexatriene" |
What You'll Learn

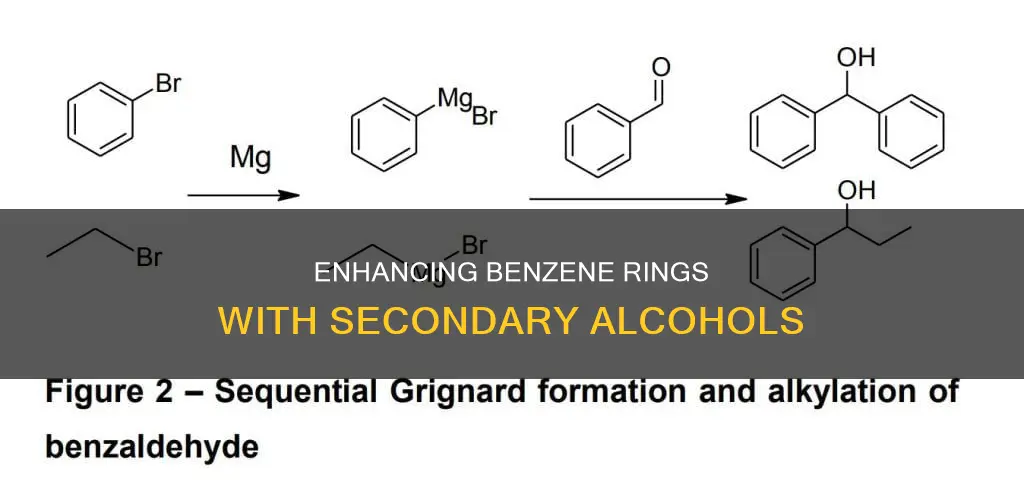
Phenyl lithium or phenyl magnesium bromide with acetone
Phenylmagnesium bromide is a Grignard reagent with the formula C6H5MgBr. It is a commercially available solution of diethyl ether or tetrahydrofuran (THF). Laboratory preparation involves treating bromobenzene with magnesium metal, often in the form of turnings. A small amount of iodine may be used to activate the magnesium to initiate the reaction.
Phenylmagnesium bromide is often used as a synthetic equivalent for the phenyl "Ph−" synthon. It can be used to obtain phenylcarbinols with a high yield. The reaction of phenylmagnesium bromide with N-sulfinyl urea/diene intermediate, followed by treatment with trimethyl phosphite, leads to the synthesis of bicyclic allylic alcohol.
Phenyl lithium, with the formula R-Li, is an organolithium reagent. It is created by reacting an alkyl halide with lithium. Both Grignard and Organolithium reagents react with ketones and aldehydes to produce alcohols. Specifically, reactions with aldehydes produce secondary alcohols.
Acetone is a ketone, and therefore, both phenylmagnesium bromide and phenyl lithium can react with acetone to produce alcohols. However, the specific reaction mechanisms and products are not readily available and may require further investigation.
Underage Drinking: Florida's Strict Alcohol Laws Explained
You may want to see also

Friedel-Crafts alkylation reaction
The Friedel-Crafts alkylation reaction is an organic coupling reaction involving an electrophilic aromatic substitution that is used for the attachment of substituents to aromatic rings.
The reaction proceeds via a three-step mechanism. Firstly, the Lewis acid catalyst (AlCl3) reacts with the alkyl halide, resulting in the formation of an electrophilic carbocation. This carbocation then attacks the aromatic ring, forming a cyclohexadienyl cation as an intermediate. The aromaticity of the arene is temporarily lost due to the breakage of the carbon-carbon double bond. Finally, the deprotonation of the intermediate leads to the reformation of the carbon-carbon double bond, restoring aromaticity to the compound. This proton goes on to form hydrochloric acid, regenerating the AlCl3 catalyst.
It is important to note that Friedel-Crafts alkylations are known for their unreliable kinetic behaviour due to their heterogeneous nature. Additionally, the reaction has some limitations. For instance, since the carbocations formed by aryl and vinyl halides are highly unstable, they cannot be used in this reaction. Furthermore, Friedel-Crafts reactions generally fail when electron-withdrawing groups are present on the ring, as the presence of these groups renders the aromatic ring less electron-rich and, therefore, less nucleophilic.
To address the unreliable kinetic behaviour, one strategy is to use an excess of the aromatic compound, which helps minimize polyalkylation. Another factor to consider is the presence of substituents on the aromatic ring. Electron-withdrawing substituents can inhibit the reaction, whereas highly activating groups (-OH, -NH2, -NHR) can react with the catalyst, slowing down the reaction.
Alcohol and Weight Loss: Friends or Foes?
You may want to see also

Using a Lewis acid
Adding a secondary alcohol to a benzene ring involves an electrophilic substitution reaction, such as the Friedel-Crafts acylation. This reaction typically involves two steps: the introduction of an acyl group to the benzene ring, followed by oxidation.
The first step involves reacting the benzene ring with an acyl chloride or acid anhydride in the presence of a Lewis acid catalyst, such as aluminum chloride (AlCl3) or iron chloride (FeCl3). This catalyst increases the reactivity of the benzene ring, allowing the acyl group (R-C=O) to attach to it through an electrophilic aromatic substitution process. The product of this step is an aryl ketone.
It is important to note that the choice of Lewis acid catalyst can impact the reaction. For example, using aluminum bromide (AlBr3) or iron bromide (FeBr3) instead of aluminum chloride or iron chloride can help avoid "scrambling" of the halides during the reaction.
The second step involves oxidizing the aryl ketone formed in the previous step to obtain the corresponding carboxylic acid. This can be achieved using various oxidizing agents, such as potassium permanganate (KMnO4), chromic acid (H2CrO4), or nitric acid (HNO3). The oxidation reaction converts the aryl ketone into a carboxylic acid, with benzoic acid (C6H5COOH) as the final product when R is hydrogen.
It is worth mentioning that direct Friedel-Crafts acylation with carboxylic acids is not effective because the carboxyl group can react with the Lewis acid, hindering the desired reaction. Therefore, acyl chlorides or acid anhydrides are typically used to introduce the acyl group initially.
Additionally, while the focus is on using a Lewis acid, it is worth noting that alternative methods exist to add a secondary alcohol to a benzene ring. For example, phenyl lithium or phenyl magnesium bromide can be added to acetone to generate the alcohol, although this reaction does not proceed beyond the formation of the tertiary alcohol.
Age Limit for Scanning Alcohol: Understanding the Rules
You may want to see also

Chlorination of benzene
Preparation of Reactants
Firstly, ensure you have the necessary reactants: benzene (C6H6) and chlorine (Cl2) gases. These gases can be prepared by heating them separately or in a mixture to a desired temperature, preferably between 100 and 200 °C. The reactants should be preheated to facilitate the reaction and ensure a steady rate of flow into the reaction chamber.
Selection of Chloride Salt Bath
The chlorination of benzene typically occurs in a molten salt bath containing metal chlorides. The specific composition of the salt bath can vary but often includes chlorides of aluminum, sodium, potassium, iron, bismuth, or zinc. For example, a eutectic mixture of aluminum chloride, sodium chloride, and ferric chloride can be used, as described in some patents.
Ratio of Reactants
Maintaining the appropriate ratio of benzene to chlorine is crucial for the chlorination process. The preferred ratio is typically between 0.5 to 1.0 parts of chlorine per part of benzene by weight. For mono-substitution, the theoretical ratio is 0.91 parts of chlorine per part of benzene. However, in practice, you may use a slightly lower ratio, such as 0.6 or 0.89 parts of chlorine per part of benzene, depending on the desired level of chlorination.
Reaction Conditions
The reaction temperature plays a vital role in the chlorination process. The reactant gases (benzene and chlorine) are passed into the molten salt bath, which is maintained at a temperature between 200 and 400 °C. The higher temperatures, such as 360 to 365 °C, tend to favor more complete reactions. The reactants are released into the salt bath through nozzles placed at a specific depth, usually a few inches below the surface, to ensure proper mixing.
Collection and Separation of Products
After the reaction, the chlorinated products are collected as vapors, condensed, and then separated by fractional distillation. The specific distillation temperatures and yields may vary depending on the reaction conditions and the degree of chlorination. The chlorinated products may include monochlorobenzene and polychlorobenzenes, with monochlorobenzene being of significant industrial importance as a solvent and reagent.
Safety Considerations
Withholding Support: Helping or Hindering an Alcoholic Family Member?
You may want to see also

Bromination of benzene
In the first step of the reaction, the catalyst polarizes the bromine molecule, resulting in the formation of a species that behaves as Br+. This polarized bromine molecule then reacts with the nucleophilic benzene ring, leading to the creation of a nonaromatic carbocation intermediate. The intermediate is more stable than a typical alkyl carbocation due to resonance but is less stable than the original benzene ring.
The second step involves the deprotonation of the carbocation intermediate. Instead of adding Br- to form an addition product, the intermediate loses H+ from the bromine-bearing carbon, resulting in a substitution product. This loss of H+ is similar to the second step of an E1 reaction. Overall, the reaction is exergonic because the stability of the aromatic ring is maintained.
It is important to note that the benzene ring is less reactive towards electrophiles than alkenes. For example, Br2 in a CH2Cl2 solution instantly reacts with most alkenes but does not react with benzene at room temperature. Therefore, a catalyst is necessary to initiate the bromination of benzene.
The bromination of benzene is a versatile reaction that can be applied to various compounds containing benzene rings, such as toluene, yielding a mixture of bromotoluene products. Additionally, the principles of electrophilic bromination are similar to those of electrophilic chlorination and iodination, where the benzene ring reacts with Cl+ and I+, respectively, to form new carbon-halogen bonds.
Hept-1-en-3-ol: A Primary Alcohol Exploration
You may want to see also
Frequently asked questions
One way to add a secondary alcohol to a benzene ring is by using phenyl lithium or phenyl magnesium bromide and adding that to acetone. Another way is through an acid-catalysed reaction of ketone with benzene to make benzyl alcohol.
The formula for an organolithium reagent is R-Li.
The formula for an alcohol is R–OH.
Examples of catalytic acids include H2SO4 and H2O/H2SO4.
Sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4) are two reducing reagents used to turn carbonyl groups into alcohols.







