Oxidation reactions are a significant aspect of organic chemistry, particularly in the context of alcohols. The oxidation of primary and secondary alcohols is a crucial process that leads to the formation of aldehydes, ketones, and carboxylic acids. The type of product formed depends on the specific alcohol being oxidized and the choice of oxidizing agent. Primary alcohols, for example, can be oxidized to aldehydes or carboxylic acids, while secondary alcohols typically react to form ketones. This process is fundamental in the preparation of various synthetic intermediates in organic chemistry.
| Characteristics | Values |
|---|---|
| Reaction | Oxidizing agent removes the hydrogen from the -OH group, and a hydrogen from the carbon atom attached to the -OH |
| Primary Alcohol | Converts to aldehydes or carboxylic acids |
| Secondary Alcohol | Converts to ketones |
| Tertiary Alcohol | Not oxidized |
| Common Oxidizing Agents | Pyridinium chlorochromate (PCC), pyridinium dichromate (PDC), Swern oxidation, Dess-Martin periodinane (DMP), sodium hypochlorite (NaClO), potassium permanganate (KMnO4), chromic acid (H2CrO4), chromium trioxide (CrO3) |
What You'll Learn

Oxidizing agents remove the hydrogen from the -OH group
When an oxidizing agent reacts with a primary or secondary alcohol, it removes the hydrogen from the -OH group. This is a key characteristic of oxidation reactions, where a C-H bond is broken and replaced with a C-O bond. The oxidizing agent gains electrons in this process, resulting in the reduction of the alcohol.
The oxidation of primary alcohols can lead to the formation of either aldehydes or carboxylic acids, depending on the reaction conditions. In the presence of certain oxidizing agents, such as pyridinium chlorochromate (PCC), primary alcohols are oxidized to aldehydes. Aldehydes can then undergo further oxidation to form carboxylic acids.
Secondary alcohols, on the other hand, are oxidized to ketones. This is a straightforward process that does not involve the formation of intermediate products. For example, when secondary alcohols like propan-2-ol are heated with an oxidizing agent, such as acidified sodium or potassium dichromate(VI) solution, ketones are produced.
Tertiary alcohols, however, are generally unaffected by oxidation reactions. This is because they lack the hydrogen atom attached to the carbon that is necessary for the formation of the carbon-oxygen double bond, which is a crucial step in the oxidation process.
The choice of oxidizing agent is crucial in these reactions. Common oxidizing agents used by organic chemists include chromium trioxide (CrO3) and chromic acid (H2CrO4). The selection of the appropriate oxidizing agent depends on the desired product and the specific reaction conditions.
Alcohol Abuse and Depression: What's the Link?
You may want to see also

Primary alcohols form aldehydes or carboxylic acids
The oxidation of primary alcohols forms aldehydes or carboxylic acids. This reaction involves the removal of a hydride equivalent from the alcohol, which is facilitated by the presence of an oxidizing agent. The oxidizing agent removes the hydrogen from the -OH group and a hydrogen atom from the carbon atom attached to the -OH group.
The specific product formed (either an aldehyde or a carboxylic acid) depends on the reaction conditions. The primary alcohol is first oxidized to an aldehyde, which is then further oxidized to a carboxylic acid. This two-step procedure is often used by organic chemists as the conditions for the direct oxidation of primary alcohols to carboxylic acids are harsh and not compatible with common protection groups.
The oxidation of primary alcohols to aldehydes can be achieved using a variety of reagents, including pyridinium chlorochromate (PCC), Dess-Martin periodinane (DMP), and chromium trioxide (CrO3). These reagents are milder than those used for the direct oxidation to carboxylic acids and do not oxidize the aldehyde further.
The oxidation of primary alcohols to carboxylic acids can be carried out on a commercial scale using O2/air and nitric acid as the oxidants. Other reagents that can be used include potassium permanganate (KMnO4) and chromium trioxide (CrO3). The reaction typically involves heating the alcohol under reflux with an excess of the oxidizing agent to ensure complete oxidation to the carboxylic acid.
Alcohol Abuse: Dementia Risk and Brain Health
You may want to see also

Secondary alcohols form ketones
The oxidation of alcohols is a significant reaction in organic chemistry. It involves the use of an oxidizing agent to remove a hydrogen atom from the -OH group and another hydrogen atom from the carbon atom attached to the -OH group. This process forms a carbon-oxygen double bond, which is characteristic of carbonyl-containing compounds such as aldehydes, ketones, and carboxylic acids.
When it comes to secondary alcohols, they are oxidized to form ketones. This means that the secondary alcohol loses a hydrogen atom from the hydroxyl group and another hydrogen atom from the carbon atom it is attached to. This results in the formation of a carbon-oxygen double bond and the creation of a ketone functional group. The general formula for this transformation is:
> Secondary Alcohol (R-CHOH-R') + Oxidizing Agent → Ketone (R-COR') + Water (H2O)
The specific oxidizing agents used for this process can vary. Some common examples include:
- Chromium trioxide (CrO3), which is reduced to H2CrO3 during the reaction.
- Chromic acid (H2CrO4), also known as Jones reagent.
- Potassium dichromate(VI) solution acidified with dilute sulfuric acid.
- Pyridinium chlorochromate (PCC), a milder alternative to chromic acid that is suitable for selective oxidation.
- Dess-Martin periodinane (DMP), which offers higher yields and less rigorous reaction conditions compared to PCC.
- Elemental bromine in the presence of a catalytic quantity of N-t-butyl-nitrobenzenesulfenamide, potassium carbonate, and molecular sieves.
- Sodium hypochlorite (bleach), which provides a cost-effective and mild oxidation method with high yields.
It is important to note that while primary alcohols can be further oxidized to carboxylic acids, secondary alcohols cannot be oxidized beyond the ketone stage. This is because further oxidation would require breaking the C-C bonds in the molecule, which demands a significant amount of energy.
Alcoholics and Al-Anon: A Powerful Combination
You may want to see also

Tertiary alcohols are not affected by oxidation
In the oxidation of primary and secondary alcohols, the oxidizing agent removes the hydrogen from the hydroxyl group and a hydrogen from the carbon atom attached to it. This results in the formation of a carbon-oxygen double bond. However, tertiary alcohols do not have a hydrogen atom attached to the carbon atom, which is necessary for the formation of the carbon-oxygen double bond.
While it is generally true that tertiary alcohols are resistant to oxidation, there is an exception. Under specific circumstances, when the tertiary alcohol is allylic, it can undergo a process known as an allylic shift. This involves the migration of the double bond, allowing for the subsequent oxidation of the alcohol. Bobbitt's reagent, similar to TEMPO, is particularly effective in this type of reaction.
It is important to note that while tertiary alcohols are not oxidized by common reagents, they can still undergo certain types of oxidation. For example, they can be burned, which is a form of oxidation. However, in the context of organic chemistry, there are important mild oxidation methods that are ineffective in oxidizing tertiary alcohols.
In summary, tertiary alcohols are generally resistant to oxidation due to their structural features, particularly the absence of a hydrogen atom attached to the carbon atom. This distinction sets them apart from primary and secondary alcohols, which can be oxidized using various reagents and conditions.
Alcohol to Minors: Felony or Misdemeanor?
You may want to see also

Common oxidizing agents include sodium or potassium dichromate(VI)
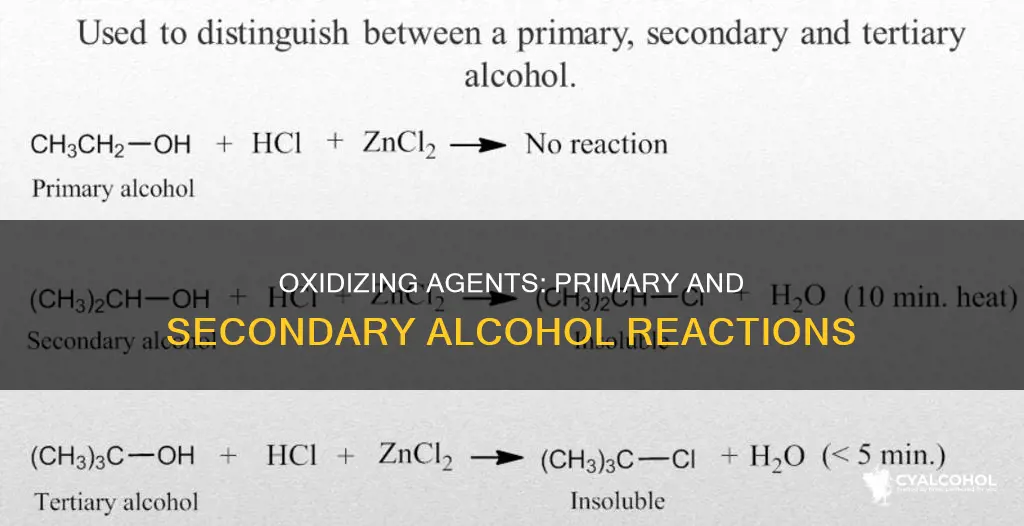
The oxidation of alcohols using acidified sodium or potassium dichromate(VI) solution is a common method to distinguish between primary, secondary, and tertiary alcohols. This reaction is also used to make aldehydes, ketones, and carboxylic acids.
The oxidizing agent used in these reactions is typically a solution of sodium or potassium dichromate(VI) acidified with dilute sulfuric acid. If oxidation occurs, the orange solution containing the dichromate(VI) ions is reduced to a green solution containing chromium(III) ions. This color change is a clear indication of the oxidation reaction.
In the case of primary alcohols, they can be oxidized to either aldehydes or carboxylic acids, depending on the reaction conditions. When oxidized, the primary alcohol first forms an aldehyde, which can then be further oxidized to a carboxylic acid. The full equation for the oxidation of ethanol to ethanoic acid is:
> $3CH_3CH_2OH + 2Cr_2O_7^{2-} + 16H+ \rightarrow 3CH_3COOH + 4Cr^{3+} + 11H_2O$
The simplified version of this equation is:
> $CH_3CH_2OH + 2 [O] \rightarrow CH_3COOH + H_2O$
For secondary alcohols, they are oxidized to ketones. An example of this reaction is when the secondary alcohol propan-2-ol is heated with sodium or potassium dichromate(VI) solution acidified with dilute sulfuric acid.
It is important to note that tertiary alcohols are not oxidized by acidified sodium or potassium dichromate(VI) solution. There is no reaction between them. This is because tertiary alcohols do not have a hydrogen atom attached to the carbon atom, which is necessary for the formation of the carbon-oxygen double bond during the oxidation process.
Alcohol Availability at Chef Mickey's Brunch
You may want to see also
Frequently asked questions
Oxidation of alcohols involves the use of an oxidizing agent to remove the hydrogen from the -OH group and a hydrogen from the carbon atom attached to the -OH group. This leads to the formation of a carbonyl group.
Common oxidizing agents include chromic acid (H2CrO4), potassium dichromate (K2Cr2O7), pyridinium chlorochromate (PCC), and sodium dichromate (Na2Cr2O7).
Primary alcohols can be oxidized to either aldehydes or carboxylic acids, depending on the reaction conditions. The oxidation of primary alcohols to aldehydes is a partial oxidation process that is easier to achieve than the complete oxidation to carboxylic acids.
Secondary alcohols are typically oxidized to form ketones rather than aldehydes or carboxylic acids. This is because the oxidation of secondary alcohols involves the loss of hydrogen atoms from the -OH group, resulting in the formation of a ketone functional group.
Tertiary alcohols do not undergo oxidation under normal conditions because they do not have a hydrogen atom attached to the carbon atom that can be removed to form a carbon-oxygen double bond.