Tert-butyl alcohol, also known as tert-butanol, is a tertiary alcohol with the formula (CH3)3COH. It is a colourless solid with a camphor-like odour that melts near room temperature. Tert-butyl alcohol is used as a solvent, ethanol denaturant, paint remover, and gasoline booster. It is also used in the production of methyl tert-butyl ether (MTBE), which is a fuel additive. MTBE can also be used to dissolve gallstones in humans. Tert-butyl alcohol has been identified in beer, chickpeas, and cassava. Its isomers include 1-butanol, isobutanol, and butan-2-ol (also known as 2-butanol). Occupational exposure to tert-butyl alcohol may occur through inhalation or dermal contact, and the general population may be exposed through ingestion of tainted food and water or inhalation of tainted air.
| Characteristics | Values |
|---|---|
| Formula | (CH3)3COH |
| Other Names | t-BuOH, 1,1-dimethylethanol, t-butanol, t-Butylalkohol, tertiary-butyl alcohol, trimethylcarbinol, trimethylmethanol |
| Type | Tertiary alcohol |
| State | Colourless solid |
| Odour | Camphor-like |
| Miscibility | Water, ethanol, diethyl ether |
| Sources | Beer, chickpeas, cassava |
| Uses | Solvent, ethanol denaturant, paint remover ingredient, gasoline octane booster and oxygenate |
| Toxicity | Liver, kidney, brain |
What You'll Learn

Tert-Butyl alcohol is a tertiary alcohol
Tert-Butyl alcohol, also known as tert-butanol, is a tertiary alcohol with the chemical formula $(CH3)3COH$ (or t-BuOH). It is a colourless solid with a camphor-like odour that melts near room temperature. Tert-butyl alcohol is the simplest tertiary alcohol and is derived commercially as a coproduct of propylene oxide production from isobutane. It can also be produced through the catalytic hydration of isobutylene or by a Grignard reaction between acetone and methylmagnesium chloride.
As a tertiary alcohol, tert-butyl alcohol has a unique structure where there is no hydrogen atom next to the hydroxy group (-OH). This structural feature makes it resistant to oxidation to carbonyl compounds. The absence of a hydrogen atom next to the hydroxy group also allows tert-butyl alcohol to follow an SN1 reaction mechanism when reacting with hydrogen chloride to form tert-butyl chloride and water. In this reaction, the relative stability of the tert-butyl carbocation enables the SN1 mechanism to be favoured over the SN2 mechanism typically observed in primary alcohols.
Tert-butyl alcohol has various industrial applications. It is commonly used as a solvent, ethanol denaturant, paint remover ingredient, and gasoline additive. In the production of gasoline, tert-butyl alcohol serves as an octane booster and oxygenate. The oxygen content of gasoline is increased by the addition of tert-butyl alcohol, resulting in improved fuel efficiency and a higher octane rating.
Additionally, tert-butyl alcohol is an important intermediate in the synthesis of other compounds. It is used to produce methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE) through reactions with methanol and ethanol, respectively. MTBE, in particular, has therapeutic applications in dissolving gallstones in humans. Tert-butyl alcohol is also involved in the production of tert-butyl hydroperoxide (TBHP) through a reaction with hydrogen peroxide.
The presence of tert-butyl alcohol has been identified in various natural sources, including beer, chickpeas, and cassava. While it has its uses and natural sources, occupational exposure to tert-butyl alcohol may occur through inhalation and dermal contact in workplaces where butanols are produced or used. The general population may also be exposed to butanols through the ingestion of tainted food and water or inhalation of contaminated air. Understanding the toxicity effects of tert-butyl alcohol and its isomers is crucial due to the potential for high exposure and the associated health risks.
Alcohol in Pressure Cookers: Safe or Not?
You may want to see also

Tert-butyl alcohol is used as a solvent
Tert-butyl alcohol, also known as tert-butanol, is a simple tertiary alcohol with the formula (CH3)3COH (or t-BuOH). It is a colourless solid with a camphor-like odour and is miscible with water, ethanol, and diethyl ether.
Tert-butyl alcohol is indeed used as a solvent, as well as an ethanol denaturant, paint remover ingredient, and gasoline octane booster and oxygenate. As a solvent, it is used in the production of methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE) through reactions with methanol and ethanol, respectively. MTBE, in particular, is a type of oxygenate added to gasoline as a fuel additive. It increases the oxygen content of gasoline, enabling it to burn more efficiently and prevent engine knocking.
Tert-butyl alcohol is also used in the production of tert-butyl hydroperoxide (TBHP) through a reaction with hydrogen peroxide. Additionally, it is used in organic chemistry as a non-nucleophilic base. Tert-butanol can be deprotonated with a strong base to form an alkoxide, such as potassium tert-butoxide, which is commonly used in organic synthesis.
Furthermore, tert-butyl alcohol has applications in solid-phase organic synthesis as a resin. It serves as an alternative to Wang resin when strong bases are employed in the synthesis process.
Tert-butyl alcohol is derived commercially as a coproduct of propylene oxide production from isobutane. It can also be produced through the catalytic hydration of isobutylene or via a Grignard reaction between acetone and methylmagnesium chloride.
Smartphone Alcohol Bath: Safe or Not?
You may want to see also

Tert-butyl alcohol is derived from isobutane
Tert-butyl alcohol, also known as tert-butanol, is a tertiary alcohol with the chemical formula (CH3)3COH. It is a simple compound that is derived commercially from isobutane as a coproduct of propylene oxide production. This derivation process can also be achieved by the catalytic hydration of isobutylene or through a Grignard reaction between acetone and methylmagnesium chloride.
Tert-butyl alcohol is a colourless solid with a camphor-like odour. It is miscible with water, ethanol, and diethyl ether. This alcohol has a wide range of applications, including its use as a solvent, paint remover ingredient, and gasoline additive. It is also used in the production of methyl tert-butyl ether (MTBE), which is added to gasoline to improve burning efficiency and prevent engine knocking.
The toxicity effects of tert-butyl alcohol and its isomers, such as n-butanol, sec-butanol, and isobutanol, are important considerations. Occupational exposure to these compounds can occur through inhalation and dermal contact in workplaces where they are produced or used. The general population may also be exposed through the ingestion of tainted food and water or inhalation of polluted air.
The metabolic pathways of MTBE, which is derived from tert-butyl alcohol, have been described in detail. Metabolic studies have identified CYP2A6 as the primary enzyme responsible for converting MTBE into tert-butyl alcohol and formaldehyde in liver microsomes. Further conversions of tert-butyl alcohol can lead to the formation of 2-methyl-1,2-propanediol and 2-hydroxyisobutyrate, which have been detected in both rat and human urine.
Alcohol After LASIK: What's the Danger?
You may want to see also

Tert-butyl alcohol is identified in food and drink
Tert-butyl alcohol, also known as tert-butanol, is the simplest tertiary alcohol, with the formula (CH3)3COH (or t-BuOH for short). It is a colourless solid with a camphor-like odour and is miscible with water, ethanol, and diethyl ether. Tert-butyl alcohol has a variety of industrial uses, including as a solvent, paint remover ingredient, and gasoline octane booster.
Tert-butyl alcohol has been identified in a variety of food and drink products, including beer and chickpeas. It is also found in cassava, which is used as a fermentation ingredient in certain alcoholic beverages. The presence of tert-butyl alcohol in these products is likely due to the natural occurrence of tert-butyl alcohol in the fermentation process or the use of cassava as an ingredient.
The identification of tert-butyl alcohol in food and drink is important for several reasons. Firstly, from a consumer perspective, it is important to be aware of the potential presence of this compound in the products we consume. While tert-butyl alcohol is generally considered safe for consumption in small quantities, there is limited data on its pharmacology and toxicology in humans and other animals. As such, understanding the potential risks and effects of consuming tert-butyl alcohol is crucial.
Additionally, the identification of tert-butyl alcohol in food and drink can have regulatory implications. In some regions, there may be specific guidelines or regulations regarding the presence of certain compounds, including tert-butyl alcohol, in food and drink products. This is particularly relevant for alcoholic beverages, as the concentration and composition of various compounds can impact their classification and taxation.
Furthermore, the identification of tert-butyl alcohol in food and drink can also have implications for product development and quality control. By understanding the presence and concentration of tert-butyl alcohol in their products, manufacturers can make informed decisions about ingredient sourcing, processing methods, and formulation adjustments to ensure consistent quality and safety.
Overall, the identification of tert-butyl alcohol in food and drink highlights the complex nature of these products and the importance of analytical techniques in ensuring consumer safety, regulatory compliance, and product quality. While tert-butyl alcohol is not a cause for significant concern at typical consumption levels, ongoing research and monitoring are crucial to fully understand its effects and impact on human health.
Disney Cruise: Food, Alcohol — What's Included?
You may want to see also

Tert-butyl alcohol has toxic effects
Tert-butyl alcohol, also known as tert-butanol, is a simple tertiary alcohol with the formula (CH3)3COH (or t-BuOH for short). It is a colourless solid that melts near room temperature and has a distinct camphor-like odour. Tert-butyl alcohol has a variety of industrial applications, including as a solvent, paint remover, and gasoline additive. While it has its uses, tert-butyl alcohol also poses certain health risks due to its toxic effects.
Firstly, tert-butyl alcohol can be irritating to the skin and eyes. This means that individuals working with this substance should take appropriate precautions to minimise direct contact with the skin and eyes. Although it is poorly absorbed through the skin, it is important to note that tert-butyl alcohol is rapidly absorbed if inhaled or ingested, which can lead to adverse health effects.
The toxic effects of tert-butyl alcohol are not fully understood, as there is limited data on its pharmacology and toxicology in humans and other animals. However, it is known that human exposure can occur through the metabolism of fuel oxygenates, which may result in health complications.
Furthermore, tert-butyl alcohol plays a role in the production of methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE). These compounds have been associated with adverse health effects, particularly when present in drinking water. While the toxicology of tert-butyl alcohol itself requires further study, its involvement in the synthesis of these potentially harmful compounds adds to the overall understanding of its toxic nature.
Overall, while tert-butyl alcohol has various industrial applications, it is important to recognise and address its toxic effects. Further research is needed to comprehensively understand the full extent of its toxicity, particularly regarding human exposure and the potential long-term health consequences. In the meantime, precautionary measures should be emphasised to minimise direct contact and exposure to tert-butyl alcohol.
Working Out with Alcohol: Safe or Not?
You may want to see also
Frequently asked questions
Tert-butyl alcohol, also known as tert-butanol, is the simplest tertiary alcohol with the formula (CH3)3COH. It is a colourless solid with a camphor-like odour and is miscible with water, ethanol and diethyl ether.
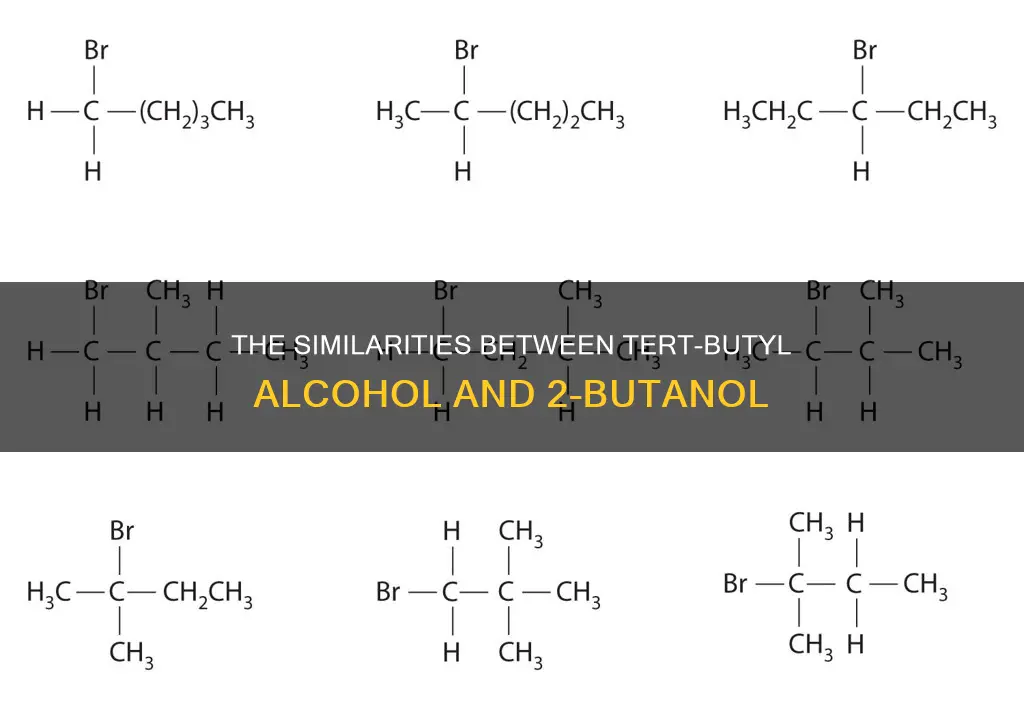
2-Butanol, also known as butan-2-ol, is an isomer of tert-butyl alcohol. It is a secondary alcohol with a hydroxy group, -OH, attached to a saturated carbon atom.
No, they are not the same compound, but they are isomers of each other, which means they have the same molecular formula but a different structure.
Tert-butyl alcohol is used as a solvent, ethanol denaturant, paint remover ingredient, and gasoline octane booster. It is also used to produce methyl tert-butyl ether (MTBE), which is a fuel additive. 2-Butanol is not commonly used but it has been identified in beer and chickpeas, and it is also found in cassava.