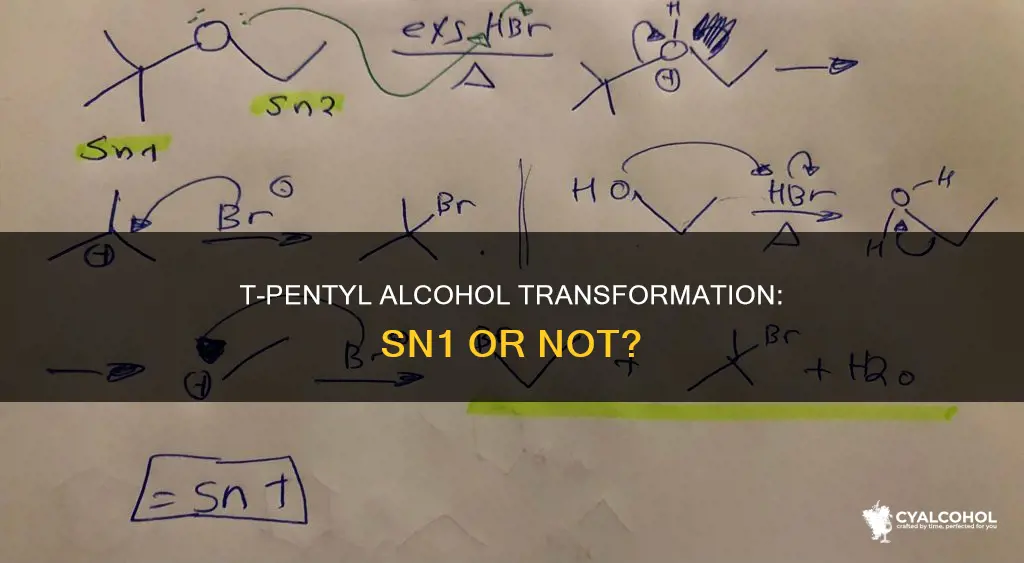
The conversion of t-pentyl alcohol to t-pentyl chloride is an SN1 reaction. SN1 reactions are a type of nucleophilic substitution that is unimolecular, meaning one bond is broken and one bond is formed in more than one step. The first step, formation of the carbocation, is slow and can be accelerated by adding a polar solvent or tertiary alkyl halide. The second step, nucleophilic attack, is faster. In this reaction, t-pentyl alcohol is treated with hydrochloric acid to produce t-pentyl chloride. The reaction is conducted in a separatory funnel, and the t-pentyl chloride separates from the aqueous reaction mixture as it is insoluble.
| Characteristics | Values |
|---|---|
| Reaction | SN1 reaction |
| Starting molecule | tert-pentyl alcohol |
| Procedure | Add ~12-13 mL of concentrated hydrochloric acid to the alcohol in the separatory funnel |
| Acid used | Hydrochloric acid (HCl) |
| Purpose | To demonstrate the procedure for the synthesis of a tertiary alkyl halide from a tertiary alcohol via the SN1 mechanism |
What You'll Learn

Tert-Pentyl Chloride Synthesis
The synthesis of tert-pentyl chloride involves the conversion of an alcohol to an alkyl halide. This reaction is carried out in the presence of an acid and halide ions, and at room temperature. Tert-pentyl chloride is an alkyl chloride with an unpleasant odour at room temperature. It is used for flavouring and odour purposes and is an isomer of 1-chloropentane (n-amyl chloride).
The synthesis of tert-pentyl chloride can be achieved through the following steps:
Step 1: Preparation of Tert-Pentyl Alcohol
The tert-pentyl alcohol is provided in a small vial, typically around 5 mL in volume. The vial is weighed, including the cap and its contents, and the mass is recorded accurately.
Step 2: Transfer to a Separatory Funnel
The alcohol is carefully poured into a separatory funnel, typically with a volume of 125 mL. A glass funnel is used during this step to avoid contaminating the neck of the separatory funnel.
Step 3: Addition of Concentrated Hydrochloric Acid (HCl)
Approximately 12-13 mL of concentrated hydrochloric acid (HCl) is added to the alcohol in the separatory funnel. Again, a glass funnel is utilised to prevent contamination. The funnel is not stoppered at this stage.
Step 4: Mixing and Pressure Release
The mixture is gently swirled within the separatory funnel for about a minute to ensure thorough mixing. After swirling, a stopper is placed securely in the funnel's neck, and it is carefully inverted without shaking. The stopcock is then immediately opened to release the pressure.
Step 5: Separation and Distillation
The reaction between tert-pentyl alcohol and hydrochloric acid results in the formation of two layers. These layers are separated, and the product mixture is distilled. This step may be necessary to remove any alkene by-products, such as 2-methyl-2-butene, formed during the reaction.
Step 6: Confirmation of Tert-Pentyl Chloride Formation
The presence of tert-pentyl chloride is confirmed by adding a silver nitrate (AgNO3) solution. The formation of a white precipitate indicates a positive test for alkyl halides. This rapid reaction with silver nitrate to produce a whitish precipitate (silver chloride) confirms the presence of tert-pentyl chloride.
The synthesis of tert-pentyl chloride is a clear demonstration of the SN1 reaction mechanism, where a tertiary alcohol reacts with a strong hydrogen halide (in this case, HCl) to form a tertiary chloride.
Bourbon County's Alcohol Laws: Legal or Not?
You may want to see also

Tertiary Alcohol to Alkyl Halide
Tertiary alcohols, such as tert-pentyl alcohol, tend to proceed through an SN1 mechanism to form alkyl halides. In this reaction, the tertiary alcohol is treated with a strong hydrogen halide (HCl) to form the alkyl halide. The chloride ion from the HCl reacts with the carbocation formed in the final step of the reaction, giving the tertiary chloride as the product.
The SN1 reaction is a type of nucleophilic substitution that is unimolecular, meaning one bond is broken and one bond is formed in more than one step. The first step involves the formation of a carbocation by the ionization of the reagent, which can be accelerated by the presence of a polar solvent or a tertiary alkyl halide. The second step is the nucleophilic attack on either side of the alpha carbon. The substitution reaction is only sensitive to the substrate concentration.
The synthesis of tert-pentyl chloride from tert-pentyl alcohol is an example of an SN1 reaction. In this reaction, tert-pentyl alcohol is allowed to react with concentrated hydrochloric acid. As the reaction proceeds, the alkyl chloride separates from the aqueous reaction mixture as it is insoluble. A small amount of an alkene, 2-methyl-2-butene, may also be produced as an elimination product. This can be removed by distilling the product mixture.
The silver nitrate test is used to confirm the presence of tert-pentyl chloride due to its rapid reaction with silver nitrate to produce a whitish precipitate, silver chloride. The formation of this precipitate is a positive test for alkyl halides.
Overall, the conversion of tertiary alcohols to alkyl halides through the SN1 mechanism is a well-studied process that provides valuable insights into nucleophilic substitution reactions and the behaviour of tertiary alcohols in synthetic organic chemistry.
Quitting Alcohol: Why Am I So Tired?
You may want to see also

SN1 Reaction Mechanism
The conversion of t-pentyl alcohol to t-pentyl chloride involves an SN1 reaction mechanism. SN1 is a type of nucleophilic substitution that is unimolecular, meaning one bond is broken and one bond is formed in more than one step. The reaction is commonly seen in secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols.
The SN1 reaction mechanism involves two steps. The first step is the ionization of the alkyl halide in the presence of aqueous acetone or ethyl alcohol, resulting in the formation of a carbocation intermediate. The stability of the carbocation depends on the substitution pattern, with tertiary carbocations being the most stable. The second step is the nucleophilic attack on either side of the carbocation, resulting in a racemic product. The nucleophilic attack occurs before the departing halide ion has moved away from the carbocation, which is why complete racemization does not take place. The rate law of the SN1 reaction is dependent only on the concentration of the substrate (alkyl halide) and not the nucleophile.
In the conversion of t-pentyl alcohol to t-pentyl chloride, t-pentyl alcohol is reacted with concentrated hydrochloric acid (HCl). The HCl protonates the hydroxyl group, allowing it to leave as a molecule of water. The chloride ion from HCl then reacts with the carbocation in the final step of the reaction to give the tertiary chloride as the product. The reaction is conducted in a separatory funnel, and as the reaction proceeds, the alkyl chloride separates from the aqueous reaction mixture.
The SN1 mechanism tends to dominate when the central carbon atom is surrounded by bulky groups, as these groups sterically hinder the alternative SN2 reaction. The presence of bulky substituents on the central carbon also increases the rate of carbocation formation due to the relief of steric strain. The SN1 mechanism can be confirmed by the silver nitrate test, which involves the rapid reaction of t-pentyl chloride with silver nitrate to produce a whitish precipitate of silver chloride.
Alcohol Laws in NC: Under 21 and Consequences
You may want to see also

Using Hydrochloric Acid
The conversion of t-pentyl alcohol to t-pentyl chloride via an SN1 reaction involves the use of hydrochloric acid. This reaction is driven by mechanical agitation and occurs in a separatory funnel. The reaction can be outlined as follows:
> t-Pentyl Alcohol + HCl → t-Pentyl Chloride + Water
To begin the reaction, approximately 5 mL of t-pentyl alcohol is poured into a 125 mL separatory funnel using a glass funnel to prevent contamination. Then, 12-13 mL of concentrated hydrochloric acid is added to the alcohol in the separatory funnel, again using a glass funnel. The mixture is gently swirled for approximately one minute to ensure thorough mixing.
Following this, the separatory funnel is carefully inverted, and the stopcock is immediately opened to release the pressure. The reaction results in the formation of two layers, which can then be separated. The insoluble alkyl halide layer, which is the product of the reaction, is isolated from the other reactants and byproducts through washing.
The crude t-pentyl chloride is washed with distilled water to remove any unreacted t-pentyl alcohol. After distillation and the addition of AgNO3 solution, a white precipitate forms due to the rapid SN1 reaction of t-pentyl chloride with silver nitrate, producing silver chloride. This positive test confirms the presence of alkyl halides.
It is important to note that t-pentyl chloride can also be formed through an E1 elimination mechanism, resulting in the formation of an alkene byproduct, 2-methyl-2-butene. This byproduct can be removed by distilling the product mixture.
DMT and Alcohol: A Safe Mix?
You may want to see also

Testing for Success
The conversion of t-pentyl alcohol to t-pentyl chloride involves an SN1 reaction. SN1 is a type of nucleophilic substitution that is unimolecular, meaning one bond is broken and one bond is formed in more than one step. The SN1 mechanism generally passes through two steps. The first step involves the formation of a carbocation by the ionization of the reagent. This step is slow and can be accelerated by adding a polar solvent or tertiary alkyl halide. The second step is the nucleophilic attack on either side of the alpha carbon.
To test for the success of the SN1 reaction in the conversion of t-pentyl alcohol to t-pentyl chloride, the following procedures can be implemented:
Weighing and Mixing the Reactants
Tert-pentyl alcohol will be provided in a small vial. Weigh the vial, including the cap and contents, and record the mass accurately. Pour the t-pentyl alcohol into a separatory funnel, using a glass funnel to prevent contamination. Weigh and record the mass of the empty vial. Add approximately 12-13 mL of concentrated hydrochloric acid (HCl) to the alcohol in the separatory funnel, again using a glass funnel.
Mixing and Separation
Gently swirl the mixture in the separatory funnel for about one minute to ensure thorough mixing. After swirling, securely stopper the funnel and carefully invert it without shaking. Then, immediately release the pressure by opening the stopcock. This process will allow for the separation of the alkyl chloride from the aqueous reaction mixture, as the alkyl chloride is insoluble.
Distillation and Precipitate Formation
Distillation may be necessary to remove any unwanted by-products, such as 2-methyl-2-butene, which can form as an elimination product. After distillation, add an AgNO3 (silver nitrate) solution. The formation of a white precipitate indicates a positive test for t-pentyl chloride. This precipitate is silver chloride, which forms due to the rapid SN1 reaction between t-pentyl chloride and silver nitrate.
Verification of SN1 Reaction
The SN1 mechanism is characterized by the formation of a carbocation intermediate. In this reaction, the chloride ion from HCl reacts with the carbocation, resulting in the formation of the tertiary chloride product. The SN1 reaction is also distinct from the SN2 reaction, exhibiting differences in reaction rates, stereochemistry, and relative rates of reaction for primary, secondary, and tertiary alkyl halides.
Ethanol: Alcoholic Beverages' Essential Ingredient
You may want to see also
Frequently asked questions
Yes.
The purpose of this reaction is to synthesise tert-pentyl chloride from tert-pentyl alcohol.
The reaction involves treating the tertiary alcohol (t-pentyl alcohol) with a strong hydrogen halide (HCl). The chloride ion from the HCl reacts with the carbocation formed in the final step of the reaction to give the tertiary chloride as the product.
The silver nitrate test is used to confirm the presence of t-pentyl chloride due to its rapid reaction with silver nitrate to produce a whitish precipitate, silver chloride.