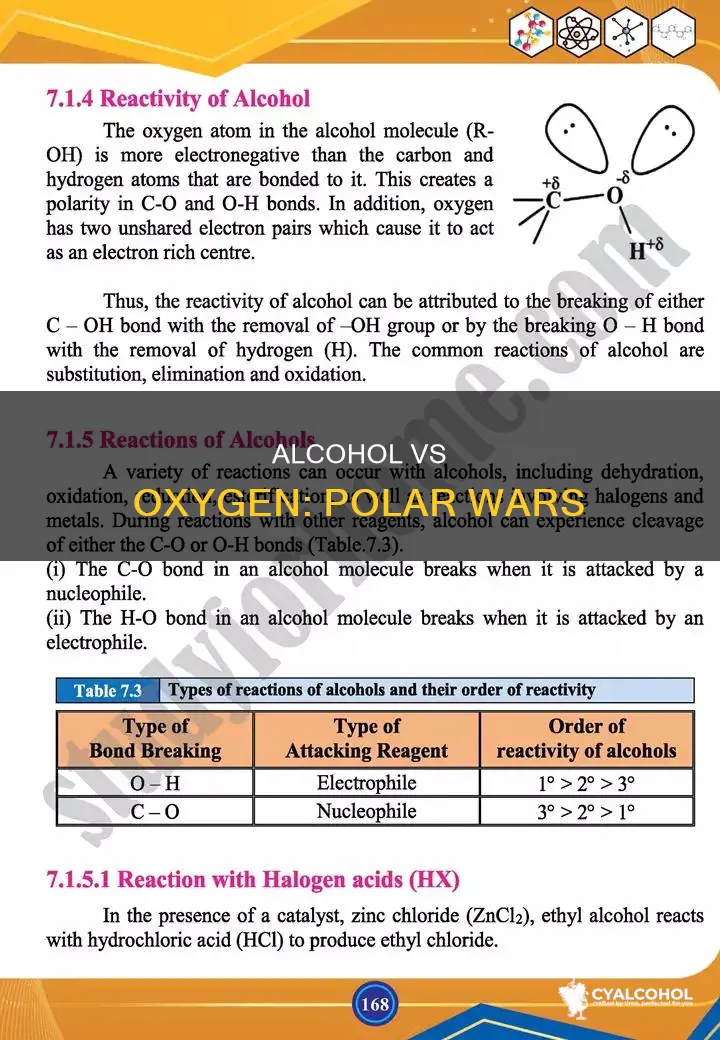
The polarity of molecules is a fundamental concept in chemistry, and understanding the polarity of substances like alcohols and carbonyls is essential. The carbonyl group, with its carbon-oxygen double bond, exhibits a high degree of polarity due to the electronegativity difference between carbon and oxygen. On the other hand, alcohols, with their single bond to oxygen and additional hydrogen, also show polarity. The question arises: which is more polar, an alcohol or a double-bonded oxygen in a carbonyl group?
| Characteristics | Values |
|---|---|
| Polarity | Alcohol is more polar than double-bonded oxygen (carbonyl) |
| Reasons | In an R-OH bond, the oxygen retains more electron density, making it more polar. |
| The carbon in a carbonyl group is electron-poor, making it an electrophile. | |
| The oxygen end of the carbonyl double bond bears a partial negative charge. | |
| The carbon-oxygen double bond is polar due to oxygen being more electronegative than carbon. | |
| The carbonyl group is susceptible to nucleophilic addition reactions. |

Resonance structures
In the context of the polarity of alcohols and carbonyls, resonance structures are a crucial concept to understand why one is more polar than the other.
In the case of the polarity of alcohols (R-OH) and carbonyls (R=O), the carbonyl group has a resonance structure where the oxygen is negatively charged and the carbon is positively charged. This is due to the presence of two lone pairs of electrons on the carbonyl oxygen, indicating the possibility of resonance. With resonance, the electrons are spread out and not concentrated, resulting in a less polar bond.
On the other hand, in an R-OH bond, the oxygen retains more electron density to itself, making it more polar. This is because the oxygen in the alcohol is also bound to hydrogen, which is less electronegative than carbon. As a result, the electron density around the oxygen in the alcohol is greater than in the carbonyl group.
Additionally, the difference in bond angles between the sp3 hybridized alcohol and the sp2 hybridized carbonyl affects their dipole moments. The vectors in the carbonyl group have more constructive addition, contributing to its polarity.
Understanding resonance structures is key to comprehending why R-OH (alcohol) is generally considered more polar than the carbonyl group.
Alcoholism and Disability: Understanding the ADA's Stance
You may want to see also

Electronegativity
The most commonly used method of calculating electronegativity was proposed by Linus Pauling in 1932, and is known as the Pauling scale (χr). This gives a dimensionless quantity, ranging from 0.79 (caesium) to 3.98 (fluorine).
The electronegativity of an atom is influenced by its atomic number and the distance at which its valence electrons are from the charged nucleus. The nuclear charge increases as you move down a group in the periodic table, but the effect of this increase is counteracted by the addition of one shell, resulting in a decrease in electronegativity as you move down the group. Fluorine, at the top of the halogen group, has the highest electronegativity, while caesium, a metal, is at the bottom with the lowest electronegativity.
The electronegativity of an atom also correlates with its first ionization energy and electron affinity. A large difference in electronegativity between two atoms leads to an ionic bond, while a smaller difference results in a covalent bond. In a polar bond, the electrons have been pulled slightly towards one end.
Now, to answer your question of whether an alcohol or double-bonded oxygen is more polar, we must consider electronegativity. A carbonyl (C=O) bond has an additional C-O bond, so some of the O atom's electron density is shared with C. In an R-OH (alcohol) bond, the oxygen retains more electron density, making it more polar. The resonance structure of a carbonyl bond also results in the oxygen being negatively charged and the carbon being positively charged, contributing to the polarity of the bond. Therefore, an alcohol or R-OH bond is more polar than a carbonyl or R=O bond.
Alcohol vs. Oil: Which Liquid Base is Best for Reed Diffusers?
You may want to see also

Nucleophilic attack
The polarity of a chemical bond is influenced by the electronegativity of the atoms involved. In the case of carbon-oxygen bonds, the double-bonded oxygen in a carbonyl group (C=O) is more polar than the single-bonded oxygen in an alcohol group (C-O). This is because the double bond is shorter, resulting in a smaller dipole moment, while the single bond with OH is longer, leading to a greater dipole moment and increased polarity. Additionally, the oxygen atom in an alcohol group is also bonded to hydrogen, which is less electronegative than carbon, allowing the oxygen to retain more electron density and contributing to its higher polarity.
Now, let's discuss nucleophilic attacks in the context of these polarities. Nucleophilic attacks play a crucial role in nucleophilic substitution reactions, which are prevalent in organic chemistry. A nucleophilic attack occurs when a nucleophile, an electron-rich species, donates its bonding electrons to an electrophile, an electron-deficient species. This process results in the formation of a new covalent bond between the two molecules. The nucleophile essentially "'attacks'" the electrophile by filling its electron deficiency with its available pair of electrons. This attack displaces the leaving group, leading to the formation of a new molecule.
The polarity of the molecules involved is crucial in nucleophilic attacks. The electron-rich nucleophile is attracted to the electron-deficient, positively charged region of the electrophile. In the context of carbon-oxygen bonds, the polarity of the double-bonded oxygen in a carbonyl group and the single-bonded oxygen in an alcohol group influences their reactivity during nucleophilic attacks. The carbonyl group, with its more polarized double bond, exhibits different reactivity patterns compared to the more polar alcohol group.
During a nucleophilic attack, the nucleophile approaches the electrophile from the backside relative to the location of the leaving group. This backside attack results in a stereochemical configuration inversion at the central carbon atom. The nucleophile donates its electrons to the electrophile, forming a new bond, while simultaneously breaking the bond between the electrophile and the leaving group. This simultaneous bond-making and bond-breaking process is known as an SN2 mechanism, with SN2 standing for substitution, nucleophilic, and bimolecular, indicating the involvement of two molecules in the rate-determining step.
The choice of solvent is also important during nucleophilic attacks. Protic solvents, such as water and alcohols, are preferred to prevent unwanted side reactions. Additionally, the nucleophile must attack an atom other than hydrogen, and the presence of bulky groups attached to the central carbon atom can interfere sterically with the SN2 reaction, influencing the reaction rate.
Alcoholism vs Alcoholic: What's the Real Difference?
You may want to see also

Dipole moment
A dipole moment is a measure of the separation of two opposite electrical charges. It occurs when there is a difference in electronegativity between two atoms involved in a bond. The greater the difference in electronegativity, the larger the dipole moment and the polarity. The dipole moment is also influenced by the distance between the charges, with the moment increasing as the distance between the charges increases.
Now, to answer your question about whether an alcohol or double-bonded oxygen is more polar, we must consider the dipole moment of each. In the case of an alcohol (ROH) molecule, the oxygen atom is more electronegative and tends to pull more electrons towards it, resulting in a higher electron density around the oxygen. This creates a dipole moment where the oxygen has a partial negative charge and the carbon has a partial positive charge. The dipole moment of the OH bond is greater than that of the carbonyl C=O bond because hydrogen is less electronegative than carbon. Therefore, the alcohol (ROH) molecule has a greater dipole moment and is more polar than the double-bonded oxygen (R=O) molecule.
Additionally, the vector components of the dipole moments should be considered. An alcohol is sp3 while a carbonyl is sp2, resulting in more constructive addition in a carbonyl than an alcohol. This also contributes to the overall polarity of the molecules.
Alcohol vs Food: Taxing Differences
You may want to see also

Reactivity
The polarity of a molecule affects its reactivity. Polar molecules have an uneven distribution of electron density, resulting in a partial negative charge on one end and a partial positive charge on the other. This polarity makes molecules susceptible to nucleophilic and electrophilic attacks.
In the context of alcohols and carbonyls, the carbonyl group (which includes aldehydes and ketones) exhibits a carbon-oxygen double bond that is highly polar. The oxygen atom in this double bond is more electronegative than carbon, resulting in a higher electron density around the oxygen atom. This polarity makes carbonyls reactive towards nucleophilic addition reactions, where nucleophiles such as alcohols, amines, or thiols can attack the carbonyl carbon. The nucleophile donates an electron pair to form a new covalent bond, and the carbonyl group's polarity makes it an attractive target for these electron-rich nucleophiles.
Alcohols, on the other hand, have an R-OH bond where the oxygen atom is bonded to both carbon and hydrogen. The electron density around the oxygen atom in an alcohol is greater than in a carbonyl because hydrogen is less electronegative than carbon. This higher electron density contributes to the overall polarity of the alcohol molecule. While alcohols can act as nucleophiles themselves in certain reactions, they are generally considered less reactive than carbonyls due to the absence of the highly reactive O-H bond.
The reactivity of aldehydes and ketones, which are types of carbonyls, also varies. Aldehydes are typically more reactive than ketones due to the lack of stabilizing alkyl groups in their structure. This higher reactivity makes aldehydes more susceptible to nucleophilic attacks and subsequent reactions.
Additionally, the concept of resonance plays a crucial role in understanding the reactivity of these molecules. Resonance structures show the distribution of electron density within a molecule. In the case of carbonyls, the resonance structure depicts the oxygen atom with a negative charge and the carbon atom with a positive charge. This polarized structure contributes to the reactivity of carbonyls by making them attractive targets for nucleophilic attacks.
In summary, the polarity and reactivity of alcohols and carbonyls are influenced by the electronegativity of the atoms involved and the resulting electron density distribution. The presence of highly polar bonds, such as the carbon-oxygen double bond in carbonyls, increases reactivity towards nucleophilic attacks. Alcohols, while also polar, exhibit lower reactivity due to the absence of certain reactive bonds. The specific structures and resonance effects of aldehydes and ketones further influence their reactivity within the broader category of carbonyls.
Alcoholism: Nature vs Nurture Debate
You may want to see also
Frequently asked questions
An alcohol (ROH) is more polar than a double-bonded oxygen (R=O) because the oxygen in the alcohol retains more electron density, making it more electronegative.
In an R-OH bond, the oxygen retains more electron density, making it more electronegative and polar. In contrast, the R=O bond has a resonance structure where the electron density is shared between the oxygen and carbon atoms, resulting in a partial negative charge on oxygen and a partial positive charge on carbon.
The resonance structure of a double-bonded oxygen, such as a carbonyl group, results in a partial negative charge on the oxygen and a partial positive charge on the carbon. This polarity makes the carbonyl group susceptible to nucleophilic and electrophilic attacks.
Nucleophiles are electron-rich atoms or molecules that donate electrons to form new covalent bonds. Electrophiles, such as carbonyl carbons, are electron-poor and attract nucleophiles. The nucleophilic addition of alcohols to carbonyl groups is a common reaction, leading to the formation of new compounds.
The length of the bond can influence the dipole moment, which is a measure of polarity. A shorter bond length, as in the case of a double bond, results in a smaller dipole moment. Therefore, a longer single bond, such as the O-H bond in alcohols, contributes to a greater dipole moment and increased polarity.







