The polarity of molecules is a fundamental concept in chemistry, influencing their physical and chemical properties. While carbonyl groups exhibit a larger dipole moment due to the highly electronegative oxygen atom, alcohol groups possess a unique advantage: their ability to form hydrogen bonds as both donors and acceptors. This capability results in stronger intermolecular forces, making alcohol groups more polar overall. This distinction becomes particularly evident when comparing compounds like carboxylic acids, which contain both carbonyl and hydroxyl groups, with alcohols. The additional hydroxyl group in carboxylic acids enhances their hydrogen bonding capacity, contributing to their higher polarity compared to alcohols. However, when considering the polarity of carbonyl and alcohol groups in isolation, the hydrogen bonding potential of alcohol groups gives them a slight edge in terms of overall polarity.
What You'll Learn

Carbonyl has a larger dipole moment
While there are differing opinions on the topic, it is generally thought that a carbonyl group has a larger dipole moment than an alcohol group, making it more polar. This is due to the electronegativity difference between the atoms in each group, with oxygen being more electronegative than both hydrogen and carbon. The carbonyl group (C=O) has a delta-+ charge on the carbon atom, which makes it more polar than the alcohol group (O-H).
However, it is important to note that the ability of alcohols to form hydrogen bonds, as both donors and acceptors, gives them stronger intermolecular forces overall, making them more polar molecules. Alcohols can dissolve well in water due to their polarity. Carboxylic acids, which contain both a carbonyl group and a hydroxyl group, are the most polar, as they can form multiple polar bonds and hydrogen bonds.
The polarity of a compound also affects its physical properties, such as boiling point and solubility. Compounds with stronger intermolecular forces, like alcohols, tend to have higher boiling points and greater solubility in water. For example, aldehydes have lower polarity than alcohols and carboxylic acids because they lack the hydroxyl group for hydrogen bonding, despite having a carbonyl group.
In certain cases, the structure of the compound may also influence its polarity. Some sources suggest that the oxygen atom in ketones is sp2 hybridized, making it more electronegative and leading to a greater dipole moment. However, others argue that the oxygen in alcohol, being sp3 hybridized, results in a greater electronegativity difference with hydrogen, contributing to its polarity.
In summary, while carbonyl groups have a larger dipole moment due to the electronegativity difference between oxygen and carbon, alcohols exhibit stronger intermolecular forces through hydrogen bonding, making them more polar molecules overall. The polarity of a compound influences its interactions with other molecules and its physical properties, such as solubility and boiling point.
Alcohol: A CNS Depressant
You may want to see also

Alcohol has stronger intermolecular forces
While carbonyl compounds are more polar than alcohols, the ability of alcohols to form hydrogen bonds means they exhibit stronger intermolecular forces (IMFs). Recall that hydrogen bonds are the strongest IMFs, and therefore matter more than the dipole moment.
Carbonyls have a larger dipole moment than alcohols, resulting in a more polar bond. However, alcohols are both hydrogen bond donors and acceptors, while carbonyls are only acceptors. This gives alcohols an advantage in terms of their overall polarity and strength of IMFs.
The difference in polarity between carbonyls and alcohols can be attributed to the electronegativity of the atoms involved. In the case of carbonyls, the focus is on the carbon-oxygen (C=O) bond, which results in a delta-plus charge on the carbon atom due to oxygen's high electronegativity. On the other hand, alcohols have an oxygen-hydrogen (O-H) group, which results in a larger electronegativity difference between oxygen and hydrogen. This difference in electronegativity contributes to the stronger IMFs observed in alcohols.
Additionally, the presence of a hydroxyl group (O-H) in alcohols facilitates hydrogen bonding, further enhancing their polarity. Carboxylic acids, which contain both a carbonyl group and a hydroxyl group, exhibit even stronger hydrogen bonding capabilities and, consequently, have stronger intermolecular forces than alcohols.
In summary, while carbonyls have a more polar bond, alcohols possess stronger intermolecular forces due to their ability to form hydrogen bonds as both donors and acceptors. This distinction between carbonyls and alcohols is an important concept in chemistry, particularly when considering the behaviour of compounds such as carboxylic acids, which exhibit even stronger hydrogen bonding and polarity due to the presence of both functional groups.
Hydrogen Peroxide vs Alcohol: Which Cleans Screens Better?
You may want to see also

Carbonyl is an H-bond acceptor
While the carbonyl group has a larger dipole moment (more polar bond) than an alcohol group, the alcohol group's ability to hydrogen bond makes it a more polar molecule overall. This is because hydrogen bonds are the strongest intermolecular force. Alcohols are both hydrogen bond donors and acceptors, whereas carbonyls are only hydrogen bond acceptors.
The carbonyl group can act as a hydrogen bond acceptor due to the presence of a polarized oxygen atom with a negative charge. This negative charge allows it to interact with donor hydrogens in other molecules. For example, the oxygen in pentanone can participate in hydrogen bonding with donor hydrogens in other molecules.
The amide nitrogen in carbonyl compounds has a lone pair of electrons and can technically function as a hydrogen bond acceptor when viewed in isolation. However, this lone pair is delocalized towards the carbonyl bond, forming a π-bond with the neighbouring carbonyl carbon. Therefore, these nitrogens are not considered good hydrogen bond acceptors.
In competitive situations between amino nitrogen and carbonyl oxygen, studies have shown that hydrogen bonds are more frequent on the oxygen than on the amino substituent. This behaviour is similar to resonance-assisted hydrogen bonding.
Stronger Alcohol: More Diuretic or Just More Fun?
You may want to see also

Alcohol is both an H-bond donor and acceptor
Alcohols are organic compounds with the functional group hydroxyl (-OH) attached to a carbon atom. The hydroxyl group includes an oxygen atom bonded to a hydrogen atom (O-H), where the hydrogen is slightly positive due to the nature of the polar bond. This makes the hydroxyl group a hydrogen bond donor.
Additionally, the oxygen atom in the hydroxyl group has two lone pairs of electrons, allowing it to act as a hydrogen bond acceptor. This ability to act as both a donor and acceptor is what gives alcohols their unique properties, such as their relatively high boiling points compared to non-polar substances of similar size.
The hydrogen (H) bond is a strong intermolecular force that plays a crucial role in various chemical and biological processes. It is formed by the interaction of a hydrogen bond donor and a hydrogen bond acceptor. The donor species is highly electronegative and has a high charge, allowing it to donate electrons to the acceptor species. The acceptor species, on the other hand, has an electron-deficient region that attracts the electron-rich donor species to form a strong partial bond.
In the context of comparing alcohol and carbonyl groups, it is important to note that while carbonyl groups are h-bond acceptors, alcohol groups are both h-bond donors and acceptors. This distinction is significant because it contributes to the overall polarity of the molecules. The ability of alcohol to form hydrogen bonds results in stronger intermolecular forces, making it a more polar molecule despite having a smaller dipole moment than carbonyl groups.
In summary, the hydroxyl group of alcohol compounds enables them to be both hydrogen bond donors and acceptors. This unique characteristic has significant implications for the properties and behaviour of alcohols, including their ability to form hydrogen bonds with themselves and their relatively high boiling points.
Alcoholism: Disability Rights in California Employment Insurance
You may want to see also

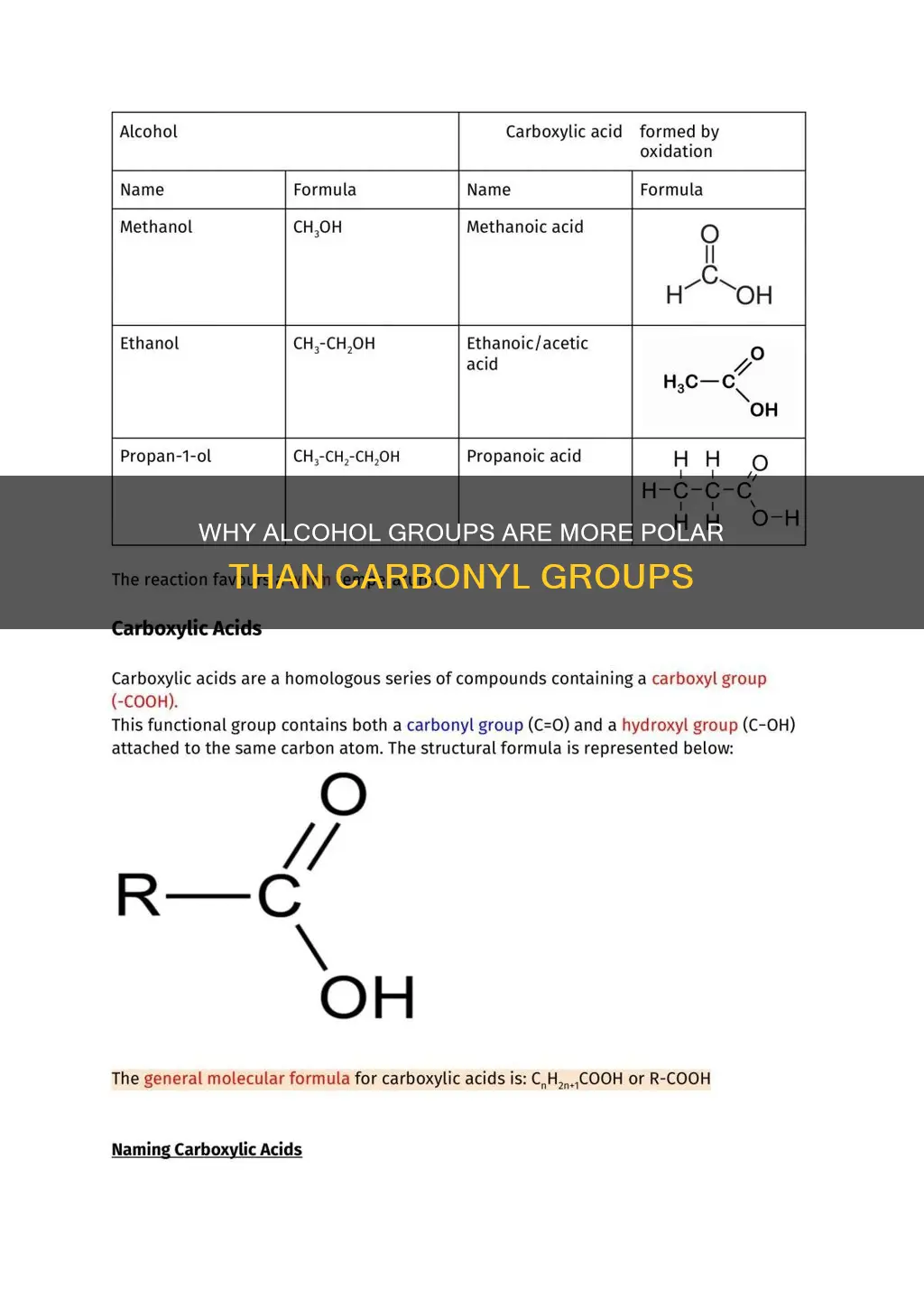
Carboxylic acid is the most polar compound
Carboxylic acids are organic acids that contain a carboxyl group. They are polar in nature due to the presence of both a carbonyl group and a hydroxyl group, which allow them to form strong hydrogen bonds. This ability to engage in hydrogen bonding is a defining characteristic of carboxylic acids and is well-documented in chemistry texts.
The polarity of carboxylic acids has significant implications for their physical properties and behaviour. For instance, smaller carboxylic acids with 1 to 5 carbons are soluble in water due to their polar nature, while larger carboxylic acids have limited solubility in water because of the increasing hydrophobic nature of the alkyl chain. However, these longer-chain acids are soluble in less polar solvents such as ethers and alcohols. Carboxylic acids also tend to have higher boiling points than water due to their greater surface areas and their propensity to form stabilised dimers through hydrogen bonds.
The polarity of carboxylic acids is utilised in various industrial applications. They are commonly found in toiletries and detergents due to their fatty acid content. For example, molecules of fatty acids are combined with glycerin to create soap through a process called saponification. Carboxylic acids are also used in the production of polymers, pharmaceuticals, solvents, and food additives.
Furthermore, carboxylic acids play a crucial role in biological systems. Amino acids, which are essential components of proteins, are considered carboxylic acids. These amino acids are vital for cell repair processes and are the primary constituents of muscles, skin, and hair. Another example of a carboxylic acid is ascorbic acid, which is a form of Vitamin C found in many common beverages and food items.
In summary, carboxylic acid is the most polar compound among the given options of alcohol, ether, and aldehyde. This polarity arises from the presence of both carbonyl and hydroxyl groups, enabling strong hydrogen bonding and dipole-dipole interactions, resulting in higher boiling points and greater solubility in water compared to the other compounds.
Alcohol in Saudi Arabia: A New Era?
You may want to see also
Frequently asked questions
Yes, alcohol groups are more polar than carbonyl groups. This is because alcohol groups can hydrogen bond, while carbonyl groups can only accept hydrogen bonds.
Polarity refers to the presence of two oppositely charged poles in a molecule. This occurs when electrons are shared between atoms in a way that creates a slight electrical charge.
Yes, carbonyl groups have a large dipole moment, which means they have a polar bond. However, they are less polar than alcohol groups.
A carbonyl group is a functional group with a double bond between a carbon atom and an oxygen atom (C=O).
An alcohol group is a hydroxyl group (-OH) attached to a carbon chain.







