Carboxylic acids are essential building blocks in organic synthesis and are used in various industries, including pharmaceuticals, polymers, and agrochemicals. Alcohols are organic compounds that can be transformed into various functional groups through chemical reactions. One such conversion is the oxidation of alcohols to carboxylic acids. This process involves using strong oxidizing agents, such as potassium permanganate (KMnO4), chromic acid (H2CrO4), or sodium dichromate (Na2Cr2O7), which provide oxygen atoms to convert the alcohol functional group (-OH) into a carboxylic acid functional group (-COOH). The reaction is typically carried out in a solvent, such as water or an organic solvent, and the progress is monitored using analytical techniques like thin-layer chromatography or spectroscopic methods. Once the oxidation is complete, the carboxylic acid is isolated and purified through methods such as crystallization or distillation.
| Characteristics | Values |
|---|---|
| Conversion equation | 3RCH2OH + 2Cr2O72- + 16H+ → 3RCOOH + 4Cr3+ + 11H2O |
| Conversion example | Ethanol into ethanoic acid |
| Conversion process | Heat under reflux with an excess of a mixture of potassium dichromate(VI) solution and dilute sulfuric acid |
| Conversion vessel | Suitable reaction vessel |
| Conversion solvent | Water or organic solvent (e.g. dichloromethane) |
| Conversion reaction | Stir or reflux the reaction mixture |
| Conversion monitoring | Thin-layer chromatography or spectroscopic methods |
| Conversion completion | Disappearance of the alcohol peak and appearance of the carboxylic acid peak in the spectra |
| Conversion quenching | Add a suitable quenching agent like water or a reducing agent such as sodium bisulfite |
| Carboxylic acid extraction | Use solvent extraction techniques |
| Carboxylic acid purification | Crystallization or distillation |
| Alternative oxidizing agents | Potassium permanganate (KMnO4), chromic acid (H2CrO4), sodium dichromate (Na2Cr2O7), pyridinium chlorochromate (PCC) |
What You'll Learn

Using oxidizing agents like potassium permanganate or chromic acid
When using oxidizing agents like potassium permanganate or chromic acid, the process differs depending on whether you are dealing with primary or secondary alcohols.
Primary Alcohols
Primary alcohols can be oxidized to either aldehydes or carboxylic acids, depending on the reaction conditions. In the case of forming carboxylic acids, the primary alcohol is first oxidized to an aldehyde, which is then further oxidized to the acid. This reaction typically occurs in two stages.
Potassium permanganate (KMnO4) is a commonly used oxidizing agent for this process. It is usually applied in an acidic, alkaline, or neutral medium. The reaction only works if there is a hydrogen attached to the carbon. It is important to note that precise control of temperature and concentrations is necessary to avoid overoxidation, which may result in the cleavage of carbon-carbon bonds.
Chromic acid (H2CrO4) is another strong oxidizing agent that can be used for the oxidation of primary alcohols to carboxylic acids. It is formed from chromium trioxide (CrO3) or sodium dichromate (Na2Cr2O7) in the presence of sulfuric acid. This reaction is known as the Jones oxidation. However, it is important to consider the drawback of producing stoichiometric amounts of chromium waste.
Secondary Alcohols
Secondary alcohols are oxidized to ketones. This conversion occurs regardless of the specific oxidizing agent used, as long as the reaction does not progress beyond the initial oxidation step.
Both potassium permanganate and chromic acid are effective oxidizing agents for converting secondary alcohols to ketones. It is important to note that secondary alcohols do not convert directly into aldehydes due to steric hindrance around the secondary carbon.
To summarize, when using oxidizing agents like potassium permanganate or chromic acid, primary alcohols can be oxidized to carboxylic acids in a two-step process, while secondary alcohols are oxidized to ketones. These reactions are valuable tools in organic chemistry for synthesizing various carbonyl compounds.
Alcohol Absorption: From Stomach to Intestines
You may want to see also

Using thionyl chloride to isolate acyl chloride
Thionyl chloride (SOCl2) is a reagent used to convert carboxylic acids to acid halides. It is also used to convert alcohols to alkyl halides. The reaction of thionyl chloride with carboxylic acids results in the formation of an acid halide, sulfur dioxide (SO2), and HCl. This reaction involves the carboxylic acid, acting as a nucleophile, attacking the electrophilic sulfur atom, leading to the replacement of hydrogen with SO2Cl.
The preparation of acyl chlorides using thionyl chloride is well-documented. One example involves adding the acid dropwise to a solution of thionyl chloride and catalytic DMF in dichloromethane over one hour, followed by stirring at 50 °C for an additional two hours. However, attempts to purify by distillation may lead to product decomposition.
Another example involves treating 1-cyano-1-cyclo-propanecarboxylic acid with thionyl chloride in refluxing benzene for two hours, resulting in the direct conversion to the acid azide. This reaction does not require a special procedure.
Thionyl chloride has also been used to prepare various thiobenzoyl chlorides from dithiocarboxylic acids, with yields of up to 80%. The reaction is analogous to the classical conversion of carboxylic acids to acyl chlorides.
The conversion of carboxylic acids to acid chlorides using thionyl chloride is valuable because it transforms the poor leaving group HO(-) into the good leaving group Cl(-). This substitution makes the carbonyl group more likely to undergo nucleophilic acyl substitution reactions. It is important to note that reactions involving thionyl chloride should be performed in a well-ventilated fume hood due to the release of foul-smelling sulfur dioxide gas.
Quitting Alcohol: Gradual Weaning or Cold Turkey?
You may want to see also

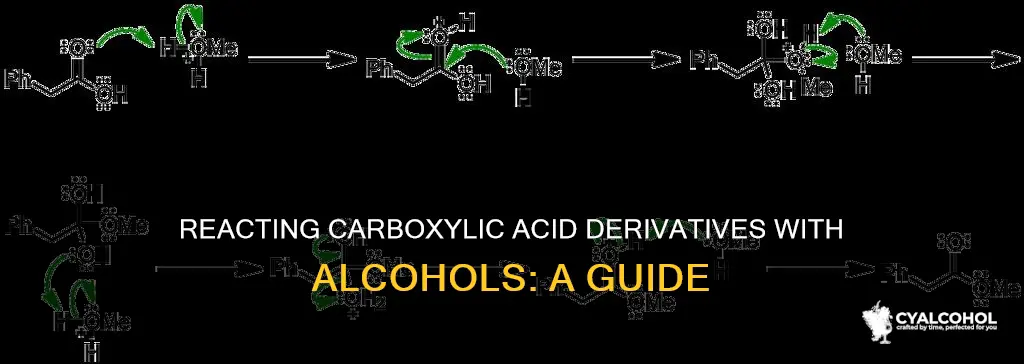
Fischer esterification
The process involves the use of sulfuric acid as a catalyst, which increases the reactivity of the carboxylic acid towards the alcohol. This step is crucial as, without it, esterification would be slow and unfavorable. To ensure a successful reaction, an excess of alcohol is used to drive the reaction towards the formation of the ester.
Safety precautions are essential when conducting Fischer esterification. In one reported incident, a researcher sustained injuries during the procedure due to a violent detonation. Therefore, it is imperative to wear appropriate personal protective equipment, including a lab coat, safety glasses, and nitrile gloves, before beginning the experiment.
The specific steps of the Fischer esterification procedure are as follows:
- Prepare a reaction mixture containing a carboxylic acid and sulfuric acid.
- Cool the reaction mixture.
- Add the desired alcohol to the cooled reaction mixture.
- Place the flask containing the reaction mixture in an oil bath preheated to the desired temperature, typically around 70°C.
- Allow the reaction to proceed for the required duration, which may vary depending on the specific reactants and conditions.
- Once the reaction is complete, isolate and purify the product as needed.
It is important to note that side reactions may occur during Fischer esterification, particularly when using unsaturated starting materials. Additionally, elevated temperatures may be necessary for reactions involving steric esters or starting materials with unreactive or steric groups.
Who Pays for Alcoholism Involuntary Commitment?
You may want to see also

Using pyridinium chlorochromate (PCC) as a mild oxidizing agent
Pyridinium chlorochromate (PCC) is a very useful reagent for the oxidation of organoboranes to carbonyl compounds in mildly alkaline and anhydrous conditions. It is a milder version of chromic acid.
PCC oxidises alcohols one rung up the oxidation ladder, from primary alcohols to aldehydes, and from secondary alcohols to ketones. It is important to note that, unlike chromic acid, PCC will not oxidise aldehydes to carboxylic acids.
When using PCC, one equivalent of the reagent is sufficient to produce the desired oxidised product. The byproducts of the reaction are Cr(IV) and pyridinium hydrochloride. The amount of water present in the reaction must be carefully controlled. If water is present, it can add to the aldehyde to create the hydrate, which could be further oxidised by a second equivalent of PCC if it is present.
In the laboratory, it is important to remember to add molecular sieves, Celite, or another solid to the bottom of the flask when using PCC.
An example of a PCC reaction is the oxidation of primary alcohols to aldehydes. This reaction involves a transfer of two electrons from the Cr to the substrate. The complete equation for this reaction is:
$$3RCH_2OH + 2Cr_2O_7^{2-} + 16H^+ \rightarrow 3RCOOH + 4Cr^{3+} + 11H_2O$$
Sneaking Alcohol on a Cruise: Possible or Myth?
You may want to see also

Converting carboxylic acids to amides or anhydrides
Carboxylic acids can be converted to amides or anhydrides through various methods. One method is to first convert the carboxylic acid into an ammonium salt, which then produces an amide when heated. The ammonium salt is formed by adding solid ammonium carbonate to an excess of the acid. For instance, ammonium ethanoate is produced by adding ammonium carbonate to an excess of ethanoic acid. The excess ethanoic acid prevents the dissociation of the ammonium salt before dehydration.
Another method involves the direct conversion of a carboxylic acid to an amide by reacting it with an amine. This can be achieved by activating the carboxylic acid to make it more reactive towards nucleophilic substitution. This activation can be done by converting the carboxylic acid into an acid chloride using reagents like thionyl chloride (SOCl2) or oxalyl chloride (C2O2Cl2). Alternatively, the carboxy OH group can be activated with a carbodiimide such as dicyclohexylcarbodiimide (DCC). The resulting activated carboxylic acid is then treated with an amine to form the corresponding amide.
In the context of converting carboxylic acids to anhydrides, one method involves the conversion of a carboxylic acid into an acid anhydride through dehydration. This process requires excessive heating (approximately 800°C) or the use of a dehydrating agent like P2O5. However, this method is only practical for acetic acid or dicarboxylic acids, as they form cyclic anhydrides.
Additionally, the conversion of an acid chloride into an acid anhydride can be achieved via an SN2 process with a carboxylate ion. This involves the nucleophilic attack of the carboxylate ion on the acid chloride, resulting in the loss of the leaving group, a chloride anion, and the formation of an acid anhydride. This technique can be used to prepare symmetrical or unsymmetrical anhydrides.
Shipping Alcohol: What's Legal and What's Not?
You may want to see also
Frequently asked questions
The first step is to set up the reaction in a suitable vessel. Add the alcohol to be oxidized and the chosen oxidizing agent, such as potassium permanganate (KMnO4) or chromic acid (H2CrO4). Stir or reflux the mixture, monitoring its progress with analytical techniques. Once the desired conversion is achieved, quench the reaction with a suitable agent, like water. Finally, extract and purify the carboxylic acid through solvent extraction and methods like crystallization or distillation.
Commonly used oxidizing agents include potassium permanganate (KMnO4), chromic acid (H2CrO4), sodium dichromate (Na2Cr2O7), and potassium dichromate (VI). The choice of agent depends on the specific reaction conditions.
Carboxylic acids are essential in organic synthesis and find wide applications in pharmaceuticals, polymers, and agrochemicals. They are also used in Fischer esterification, a reaction that produces esters.







