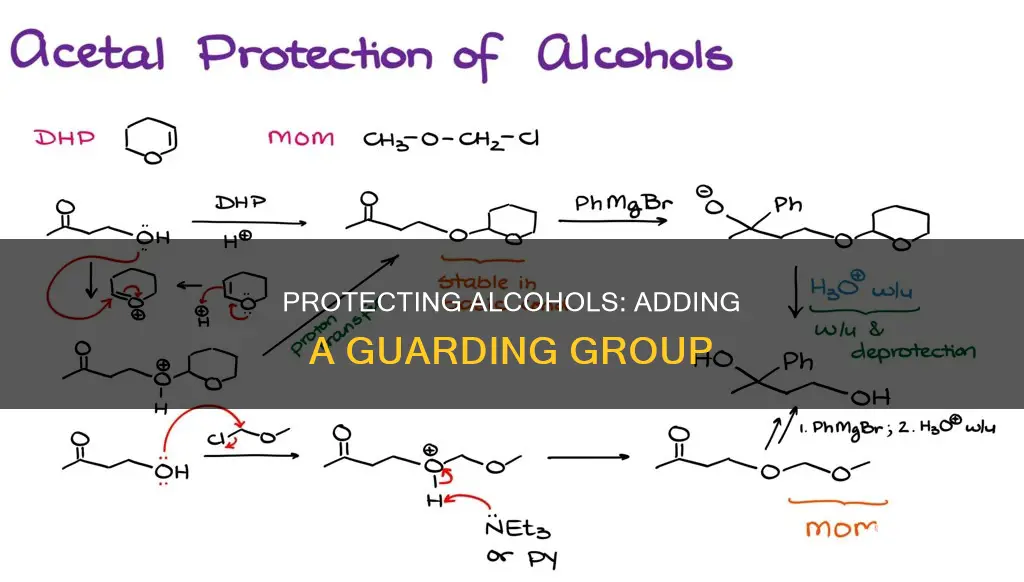
Alcohol protecting groups are an essential tool in organic synthesis, used to shield reactive functional groups from unwanted reactions. This process, known as functional group protection, involves blocking the interfering functionality by introducing a protecting group, performing the intended reaction, and then removing the protecting group to regenerate the original functional group. Protecting groups are particularly useful when multiple functional groups are present, allowing for selective reactions to occur. For example, when an alcohol and an alkyl halide coexist, a protecting group can prevent the alcohol from reacting with strong bases, enabling desired substitution reactions. One common method for protecting the hydroxy group of an alcohol involves reacting it with a chlorotrialkylsilane, Cl-SiR3, to form a trialkylsilyl ether, R'O-SiR3. Another popular protecting group for alcohols is the silyl ether group, which is stable under various reaction conditions and can be easily removed using fluoride ions or mild acids. However, the choice of protecting group must be carefully considered to ensure compatibility with other functional groups in the molecule and to maintain stability throughout the synthesis process.
| Characteristics | Values |
|---|---|
| Definition of a protecting group | A reversibly formed derivative of an existing functional group in a molecule |
| Purpose of a protecting group | To temporarily decrease reactivity so that the protected functional group does not react under synthetic conditions to which the molecule is subjected in one or more subsequent steps |
| When to use a protecting group | When there is more than one functional group on a molecule and you want to react with one and not the other |
| Protecting group for alcohol | Silyl ethers, TBS (t-butyldimethylsilyl), THP (tetrahydropyranyl) ethers, benzyl ethers, Boc-protected amino group, Fmoc-protected amino group |
| Advantages of silyl ethers | Easily cleavable, stable under a variety of reaction conditions, resistant to strong bases and nucleophiles |
| Disadvantages of silyl ethers | Sensitive to strong acids, potential for steric hindrance in bulky silyl ethers |
| How to add a silyl ether protecting group | Reacting with a chlorotrialkylsilane, Cl-SiR3, in the presence of a base such as triethylamine (TEA) or imidazole |
| How to remove a silyl ether protecting group | Reacting with an aqueous acid or fluoride ion (F-) |
What You'll Learn
- Silyl ethers are popular alcohol protecting groups due to their stability and ease of removal
- Trimethylsilyl ethers are unreactive towards many reagents and can be removed by reacting with an aqueous acid
- TBS is installed the same way as TMS, using TBSCl in the presence of a base
- THP ethers are installed by adding dihydropyran in the presence of a strong acid
- Protecting groups are used when multiple functional groups are present, allowing selective reactions

Silyl ethers are popular alcohol protecting groups due to their stability and ease of removal
Silyl ethers are a popular choice for alcohol protection due to their stability and ease of removal. They are easily formed by treating alcohols with R3SiCl in the presence of a base. The Si-F bond is unusually strong, even stronger than Si-O, and the addition of a source of fluoride ion (F-) will lead to the cleavage of Si-O bonds without affecting the rest of the molecule. A typical source of fluoride ion is the salt tetrabutylammonium fluoride (TBAF).
The stability of silyl ether can be adjusted by altering the substituents on the silicon atom. This allows for the protection of multiple alcohol groups in the same molecule, which can then be sequentially deprotected. This is a common strategy in natural product syntheses.
Silyl ethers are inert to Grignard reagents, strong bases, and oxidants, although they will be removed with strong acids. This makes them a good alternative to ordinary ethers, which are generally not used as protecting groups since their removal requires harsh conditions.
The most common silyl ether used is trimethylsilyl (TMS), although there are many others. The bulkier the groups around the silicon, the harder it is to cleave the O-Si bond. Other common silyl protecting groups include tert-butyldimethylsilyl (TBS) and Triisopropylsilyl ether (TIPS).
Silyl ethers are easily cleavable and can be removed with aqueous acid or fluoride ion, with the latter being the most common method.
Alcoholism in the Family: Kyle, Kim, and Kathy's Mother
You may want to see also

Trimethylsilyl ethers are unreactive towards many reagents and can be removed by reacting with an aqueous acid
Protecting groups are compounds that temporarily convert a given functional group into another, allowing for performing reactions that are otherwise incompatible with that functional group. They are the chemical equivalent of painter's tape.
Alcohols are highly reactive and can be converted into ethers quite easily. However, it is difficult to cleave off the ether group. Ethers are quite stable and are used as solvents for organolithium reactions. For example, in the Grignard reaction lab, diethyl ether is used.
Ethers are widely inert to a lot of conditions and are commonly used as solvents. Ether cleavage generally requires strong acid and heat, which are forcing conditions. Aqueous solutions of HBr or HI (but not HCl) tend to cleave ethers into alcohol and an alkyl halide product by either an SN2 or SN1 mechanism.
Trimethylsilyl ethers (TMS) are a type of silyl ether protecting group. The main advantage of silyl ethers is that they are easily cleavable. The Si-F bond is unusually strong – even stronger than Si-O. The addition of a source of fluoride ion (F-) will lead to cleavage of Si-O bonds without affecting the rest of the molecule. A typical source of fluoride ion is the salt tetrabutylammonium fluoride (TBAF).
Thus, trimethylsilyl ethers are unreactive toward many reagents and can be removed by reacting with an aqueous acid.
Alcohol Transportation: Interstate Legalities and Restrictions
You may want to see also

TBS is installed the same way as TMS, using TBSCl in the presence of a base
Protecting groups are used for alcohols in a variety of different situations. One of the most common protecting groups is the trimethylsilyl (TMS) group. However, TMS groups are extremely labile, especially on a phenol hydroxyl group. As such, TBS (tert-butyldimethylsilyl) is often used as an alternative.
TBS is a protecting group for alcohols and is installed the same way as TMS, using TBSCl in the presence of a base. TBSCl forms hygroscopic white crystals in DMF with imidazole or DMAP. The classic mechanism contains the generation of an imidazolium intermediate, which acts as a silyl transfer reagent for the actual silylation. TBS is more stable than TMS and can be deprotected with fluoride anions (e.g., TBAF) or strong acids.
The general procedure for silylating alcohols involves reacting the alcohol with a silyl chloride and an amine base. The Corey protocol is a reliable and rapid procedure where the alcohol is reacted with a silyl chloride and imidazole at a high concentration in DMF.
TBS protection is particularly useful when selective protection is required based on steric hindrance. TBS can also be used in conjunction with TMS to protect different hydroxyl groups within the same molecule. For example, in the synthesis of (+)-heilonine, TBS was used to protect the primary alcohol, while TMS was used to protect the tertiary hydroxyl group.
The choice between using TMS and TBS depends on the specific requirements of the synthesis. While TBS is bulkier and more stable than TMS, it may also be more challenging to introduce to the molecule.
Alcoholism and Marriage: Staying or Leaving?
You may want to see also

THP ethers are installed by adding dihydropyran in the presence of a strong acid
Protecting groups are used as a temporary block to a given functional group, allowing for reactions that would otherwise be incompatible with that functional group. In the context of alcohol protection, THP ethers are a useful group of protecting agents.
THP, or tetrahydropyran, is an organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. THP ethers are derived from the reaction of alcohols and 3,4-dihydropyran. THP ethers are installed by adding dihydropyran in the presence of a strong acid. The dihydropyran ring is activated by the strong acid, protonating the enol ether at carbon, which results in an oxonium ion. This oxonium ion is then attacked by the alcohol to give the ether.
The use of strong acid in THP protection can protonate the OH group, resulting in the loss of water to form a tertiary carbocation. This is a stable molecule, but the rate of attack of the tertiary alcohol at the electrophile may be slow.
THP ethers are a good alternative to silyl ethers, which are the most common protecting group for alcohols. THP ethers are stable under basic conditions but can be cleaved with an acid. They are formed under solvent-free conditions and are catalysed by bismuth triflate, a relatively non-toxic catalyst.
In summary, THP ethers are a useful protecting group for alcohols, installed by adding dihydropyran in the presence of a strong acid. The process involves the protonation of the enol ether, forming an oxonium ion, which is then attacked by the alcohol to give the ether.
Halal Foodies: Alcohol in Food, Safe to Eat?
You may want to see also

Protecting groups are used when multiple functional groups are present, allowing selective reactions
Protecting groups are essential when multiple functional groups are present, enabling selective reactions by blocking the reactivity of specific functional groups. They act as temporary chemical frameworks, allowing synthetic modifications to be carried out on other groups. This is particularly useful in small-scale laboratory work and initial development, where the introduction of protecting groups is relatively straightforward.
In the context of adding an alcohol-protecting group to an OH group, the protection of the OH group can be achieved through the use of silyl ethers. Silyl ethers are favoured over plain ethers due to their ease of cleavage. The Si-F bond in silyl ethers is stronger than the Si-O bond, and the addition of a fluoride ion (F-) source will lead to the cleavage of Si-O bonds without disrupting the rest of the molecule. A typical fluoride ion source is the salt tetrabutylammonium fluoride (TBAF).
Another option for protecting an OH group is the use of tetrahydropyranyl (THP) ethers. THP ethers are formed by adding dihydropyran (an "enol ether") in the presence of a strong acid. The strong acid protonates the enol ether at the carbon, resulting in an oxonium ion. This oxonium ion is then attacked by the alcohol, leading to the formation of the ether protecting group.
The choice between silyl ethers and THP ethers depends on the specific requirements of the reaction and the stability of the protecting group under different conditions. For example, THP ethers are stable under basic conditions but can be cleaved with an acid. On the other hand, silyl ethers are easily cleavable with fluoride ions.
In cases where multiple functional groups are present, selective protection can be achieved through different reactivity profiles. For instance, a Boc-protected amino group can be deprotected in acidic media, while an Fmoc-protected amino group can be deprotected under basic conditions. This allows for the selective deprotection of one functional group while leaving the other protected, enabling further reactions on the deprotected group.
Alcohol in Cars: What's the Law in New Zealand?
You may want to see also
Frequently asked questions
A protective group, or a protecting group, is a reversibly formed derivative of an existing functional group in a molecule. It is temporarily attached to decrease reactivity so that the protected functional group does not react under synthetic conditions to which the molecule is subjected in one or more subsequent steps.
Protecting groups are essential in organic synthesis to shield reactive functional groups, or moieties, from unwanted reactions. They are used when multiple functional groups are present, allowing selective reactions. For instance, when an alcohol and an alkyl halide coexist, a protecting group can prevent the alcohol from reacting with strong bases like alkynides, enabling desired substitution reactions.
Silyl ethers are popular protecting groups for alcohols due to their stability under a variety of reaction conditions and their ease of removal. Advantages include their resistance to strong bases and nucleophiles, making them suitable for reactions that require such conditions. Trimethylsilyl ethers, tert-butyldimethylsilyl (TBS), and Triisopropylsilyl ether (TIPS) are some examples of silyl ethers. Tetrahydropyranyl (THP) is another example of a protecting group.
The process of functional group protection involves three steps: blocking the interfering functionality by introducing a protecting group, performing the intended reaction, and removing the protecting group to reform the original functional group. The nature of the protective group must be chosen carefully to ensure adequate stability throughout all the intermediary synthesis steps.