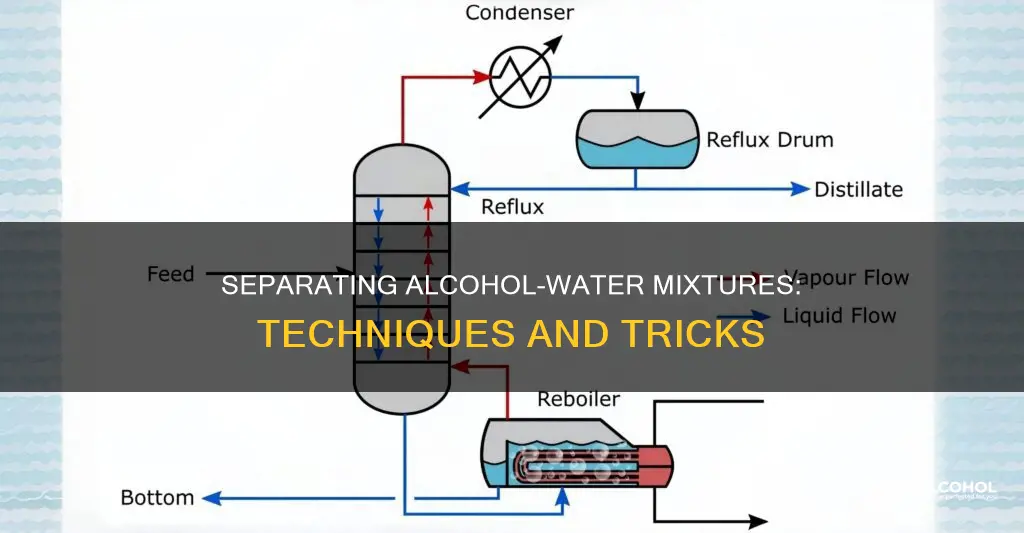
There are several methods to separate a mixture of alcohol and water. The most common method is distillation, which involves heating the mixture to a specific temperature, causing the alcohol to evaporate and condense separately from the water. This method can be enhanced by using a fractional column to trap the less volatile gases and improve the separation. Another approach is freezing, which allows for the partial removal of non-alcoholic components, resulting in a mixture richer in alcohol. Additionally, salt can be used to separate isopropyl alcohol from water, as it bonds with water and causes alcohol molecules to separate and form a second layer of liquid.
| Characteristics | Values |
|---|---|
| Boiling points | Alcohol: 78°C, Water: 100°C |
| Separation methods | Heating, freezing, distillation, salting out, molecular sieve, chromatography |
| Salt separation method | Salt bonds with water, allowing alcohol to separate and form a second layer of liquid |
| Gravity separation method | Salt settles to the bottom of the jar, liquid rising to the top will be higher in alcohol |
What You'll Learn

Heat the mixture to 80°C (176°F)
Heating a mixture of alcohol and water to 80°C (176°F) is a common method used to separate the two substances. This process is known as distillation and takes advantage of the different boiling points of alcohol and water. With a boiling point of 100°C (212°F), water requires higher temperatures to evaporate compared to alcohol, which boils at 78°C (172°F).
To perform the distillation, the mixture is heated in a round-bottomed flask, a standard piece of laboratory equipment. As the temperature rises, the alcohol, being more volatile, will begin to evaporate first. This vapour can then be directed into a condenser, where it cools and transforms back into a liquid, ready for collection in a separate container.
The use of a fractionating column attached to the flask can enhance the separation process. This glass cylinder, lined with metal rings or glass/plastic beads, helps trap less volatile gases at lower levels. As the vapour rises, only the most volatile substance, in this case, alcohol, reaches the top.
Distillation is a versatile technique with applications beyond just the separation of alcohol and water. It is used in the production of distilled beverages, desalination, the petroleum industry, and even the separation of air into components like oxygen and nitrogen.
While heating the mixture to 80°C is an effective method, it is important to note that other techniques exist, such as freezing the mixture or using salt to separate specific types of alcohol from water.
Alcohol Return Laws in New York: What's the Verdict?
You may want to see also

Use a fractionating column
To separate a mixture of alcohol and water, fractional distillation is a commonly used method. This technique involves heating the mixture and using a fractionating column to separate the two liquids based on their different boiling points.
The first step in the process is to heat the mixture of alcohol and water in a round-bottomed flask to a temperature of around 80°C (176°F). The boiling point of water is 100°C (212°F), while the boiling point of alcohol is 78°C (172°F), so by heating the mixture to a temperature between these two values, we can ensure that the alcohol will evaporate first.
The next step is to attach a fractionating column to the flask. The fractionating column is a straight glass cylinder that is lined with metal rings or glass/plastic beads. These rings or beads help trap the less volatile gases at the lower levels of the column. As the vapour rises from the distilling liquid, only the most volatile liquid (in this case, alcohol) rises to the top.
The fractionating column is designed to be hotter at the bottom and cooler at the top. This temperature gradient allows the alcohol vapour to condense back into a liquid as it rises through the column. The liquid alcohol then drops into a collection vessel attached to the top of the column.
It is important to control the temperature of the column carefully to ensure that the water vapour formed during the heating process condenses within the column and drops back into the flask. This prevents water from contaminating the pure alcohol collected at the top of the column.
By using a fractionating column, the ethanol produced will be pure and should not contain any water. This method allows for the separate collection of both ethanol and water, with the ethanol reaching an industry-standard purity level of 95% with the remaining 5% being water.
Age Limit for Alcohol Consumption in Georgia
You may want to see also

Use salt to separate isopropyl alcohol
There are several methods for separating alcohol and water. The most common method is to heat the blended liquid. Since alcohol has a lower boiling point than water, it will rapidly turn to steam. This vapour can then be condensed into a separate container.
Another method is to freeze the mixture, which allows for the partial removal of the non-alcoholic components. The remaining liquid will be richer in alcohol.
A third method is to use salt. Salt can be used to separate isopropyl alcohol from water through a process called "salting out" or "salt-induced phase separation". This involves adding salt to a mixture of isopropyl alcohol and water, then shaking the mixture vigorously. The salt competes with the alcohol in binding to the water molecules. This is because salt is an ionic compound, meaning it is made up of electrically charged molecules called ions. When dissolved in water, these ions separate and are surrounded by water molecules in a process called solvation. Because salt ions are charged, they attract water molecules more strongly than alcohol molecules, as alcohol is less polar than water. Therefore, when there is a high concentration of salt, all the water molecules will bond to the salt ions, leaving none to bond with the alcohol molecules. As a result, the alcohol becomes immiscible with water and forms a separate layer.
- Mix isopropyl alcohol and water in a bottle to create a homogeneous liquid.
- Add salt to the mixture. Ordinary table salt can be used, but ensure it is non-iodized.
- Secure the lid on the bottle tightly and shake the mixture vigorously for 20 to 30 seconds. Ensure the salt is well combined with the liquid.
- Allow gravity to separate the contents of the mixture. This will take 15 to 30 minutes. The salt will settle to the bottom of the jar, and the liquid rising to the top will be higher in alcohol.
- Carefully open the jar to avoid excess shaking, which will disturb the salty contents at the bottom.
- Use a baster to extract the distilled alcohol from the top of the mixture and transfer it to a labelled container.
Alcohol Sales at MSG During Big Ten Tournament
You may want to see also

Freeze the mixture
To separate a mixture of alcohol and water, you can freeze the mixture. This method is known as freeze distillation or fractional freezing. It relies on the different freezing temperatures of alcohol and water, just as heat distillation relies on their different boiling temperatures. Freeze distillation has been used since the 7th century and is sometimes called the Mongolian still.
To freeze distill a mixture of alcohol and water, start with a liquid that is 5%-15% alcohol. You will need a container that can be safely frozen and thawed, and a freezer or outdoor temperatures that are below 0 °C (32 °F). As water expands when it freezes, make sure your container is large enough to hold the expanded liquid without bursting. Place the alcoholic liquid into the container and put it in the freezer.
Once the mixture is frozen, remove the container from the freezer. The frozen material will be mostly water, while the alcohol, which has a higher freezing temperature, will be left behind in liquid form. Siphon out the liquid alcohol from the solid water in the container. The remaining liquid will be higher in alcoholic content, though not pure alcohol. It will also have a stronger flavour. This method is a popular distillation technique for hard apple cider (or apple jack), ale, or beer.
It is important to note that freeze distillation does not remove impurities like heat distillation does. In fact, fractional freezing may increase the ratio of impurities to the total volume of the beverage, which can cause side effects for the drinker, such as intense hangovers and a condition known as "apple palsy".
Quitting Alcohol: Why Do I Still Feel Tired?
You may want to see also

Use filtration, evaporation, or chromatography
The processes of filtration, evaporation, and chromatography can be used to separate a mixture of alcohol and water. These methods are based on differences in physical properties, such as boiling points, and the affinity of the mixture's components for a mobile or stationary phase.
Filtration
Filtration is a physical separation method that involves passing a mixture through a filter, which can be a membrane, a paper, or a porous solid, to separate the components based on their size or state of matter. For example, in a mixture of sand and salt, filtration can be used to remove the sand, and evaporation can then be applied to the salt solution to retrieve salt crystals.
Evaporation
Evaporation is another physical separation technique that involves heating a mixture to convert it into a vapour state, which is then condensed back into a liquid. This method is best suited for separating liquids with different boiling points, such as alcohol and water. Alcohol has a lower boiling point of 78°C compared to water's boiling point of 100°C. Therefore, when the mixture is heated, the alcohol evaporates first and can be condensed into a separate container.
Chromatography
Chromatography is a versatile separation technique that can be used for mixtures of solids, liquids, or a combination of both. It involves a stationary phase, which can be a solid adsorbent substance coated on glass plates, and a mobile phase, which is a fluid placed in a developing tank. The mixture is placed on the stationary phase, and the mobile phase passes through, carrying the components of the mixture at different rates depending on their affinity for the mobile or stationary phase. This separation technique is particularly useful for identifying and purifying small molecules, such as amino acids, carbohydrates, and fatty acids.
In summary, the separation of a mixture of alcohol and water can be achieved through various methods, including filtration, evaporation, and chromatography. The choice of method depends on the specific mixture and the physical properties of its components.
Alcohol Detox: One Week to a Sober You
You may want to see also
Frequently asked questions
The simplest method is to heat the mixture to 80°C (176°F). Since alcohol has a lower boiling point than water, it will quickly evaporate and can be condensed into a separate container.
You will need a heat source, such as a heating mantle or bunsen burner, and a round-bottomed glass flask (or boiling flask) to heat the mixture in. You will also need a second glass container to collect the evaporated alcohol.
Yes, there are a few alternative methods. One way is to freeze the mixture, which will allow for the partial removal of non-alcoholic components. You can also use salt to separate isopropyl alcohol from water, as salt bonds with water rather than alcohol.
The process is called distillation.
Yes, the same methods can be applied to separate alcohol from alcoholic drinks. However, it is important to note that most of the "flavorful" organic compounds will partition into the ethanol and be lost during the separation process.







